
(S)-1-(2-(tert-butyldimethylsilyloxy)-1-(4-chloro-3-fluorophenyl)ethyl)-4-(2-(methylthio)pyrimidin-4-yl)pyridin-2(1H)-one synthesis
- Product Name:(S)-1-(2-(tert-butyldimethylsilyloxy)-1-(4-chloro-3-fluorophenyl)ethyl)-4-(2-(methylthio)pyrimidin-4-yl)pyridin-2(1H)-one
- CAS Number:1453851-79-0
- Molecular formula:C24H29ClFN3O2SSi
- Molecular Weight:506.11
![Benzenemethanol, 4-chloro-α-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-3-fluoro-, 1-methanesulfonate, (αR)-](/CAS/20210305/GIF/1453851-59-6.gif)
1453851-59-6
0 suppliers
inquiry
![2(1H)-Pyridinone, 4-[2-(methylthio)-4-pyrimidinyl]-](/CAS/20180703/GIF/1453851-57-4.gif)
1453851-57-4
21 suppliers
$130.00/500mg

1453851-79-0
3 suppliers
inquiry
Yield:1453851-79-0 63%
Reaction Conditions:
Stage #1: 4-(2-(methylthio)pyrimidin-4-yl)pyridin-2(1H)-onewith potassium hexamethylsilazane in tetrahydrofuran at 0; for 0.166667 h;
Stage #2: (R)-2-((tert-butyldimethylsilyl)oxy)-1-(4-chloro-3-fluorophenyl)ethyl methanesulfonate in tetrahydrofuran at 20; for 30 h;
Steps:
A
[00252] Step A: 1.0M KHMDS (5.09 mL, 5.09 mmol) as a solution in THF was added to a cold (0°C) suspension of 4-(2-(methylthio)pyrimidin-4-yl)pyridin-2(lH)-one (0.93 g, 4.24 mmol) in THF (20 mL). The reaction mixture was stirred at 0°C for 10 minutes before (Z?)-2-(fert-butyldimethylsilyloxy)- 1 -(4-chloro-3-fluorophenyl)ethyl methanesulfonate (2.44 g, 6.36 mmol) was added as a solution in THF (5 mL). The reaction was heated to reflux for 30 hours and then cooled to room temperature and concentrated. The residue was taken up in ethyl acetate (200 mL) and washed with water. The organics were dried, filtered and concentrated. The crude product was purified via column chromatography, eluting with hexanes/ethyl acetate (4:1) to give (S)-l-(2-(tert-butyldimethylsilyloxy)-l-(4-chloro-3- fluorophenyl)emyl)-4-(2-(memylthio)pyrimidin-4-yl)pyridin-2(lH)-one (1.35 g, 63%) as a solid. 1H NMR (400 MHz, CDC13) δ 8.66 (d, J=5.0 Hz, 1H), 7.43 (m, 2H), 7.34 (d, J=5.0 Hz, 1H), 7.32-7.28 (m, 2H), 7.16 (m, 1H), 6.85 (m, 1H), 6.24 (m, 1H), 4.35 (m, 1H), 4.23 (m, 1H), 2.65 (s, 3H), 0.88 (s, 9H), 0.03 (s, 3H), -0.03 (s, 3H); m/z (APCI-pos) M+l = 506.1, 508.1.
References:
WO2013/130976,2013,A1 Location in patent:Paragraph 00252

1453854-84-6
22 suppliers
inquiry

1453851-79-0
3 suppliers
inquiry
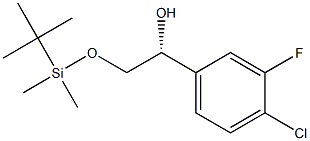
1395078-43-9
12 suppliers
inquiry

1453851-79-0
3 suppliers
inquiry
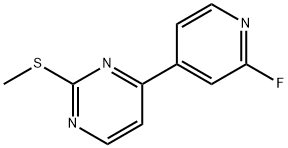
1453851-73-4
14 suppliers
inquiry

1453851-79-0
3 suppliers
inquiry
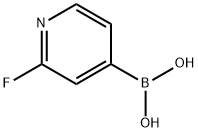
401815-98-3
242 suppliers
$10.00/250mg

1453851-79-0
3 suppliers
inquiry