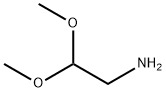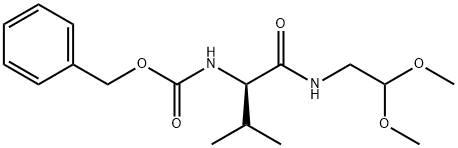
(R)-benzyl (1-((2,2-dimethoxyethyl)amino)-3-methyl-1-oxobutan-2-yl)carbamate synthesis
- Product Name:(R)-benzyl (1-((2,2-dimethoxyethyl)amino)-3-methyl-1-oxobutan-2-yl)carbamate
- CAS Number:1456693-00-7
- Molecular formula:C17H26N2O5
- Molecular Weight:338.4
Yield:1456693-00-7 99.5%
Reaction Conditions:
Stage #1: N-[(benzyloxy)carbonyl]-D-valinewith 4-methyl-morpholine;isobutyl chlorocarbonate in tetrahydrofuran at -15; for 0.5 h;
Stage #2: 2,2-dimethoxyethylamine in tetrahydrofuran at -15; for 2 h;
Steps:
2.1
Step 1: A solution of Cbz-D-Valine (500 g, 1.99 mol) and N-methylmorpholine (201.8 g, 1.99 mol) in anhydrous THF (8 L) was cooled to -15 °C and /-butyl chlorofomate (299 g, 2.19 mol) was added dropwise under stirring. After 30 min, a solution of l-amino-2,2- dimethyoxyethane (209.5 g, 1.99 mol) in THF (1 L) was added slowly and the temperature was maintained at -15 °C for 2 h. The reaction mixture was washed with brine (2 L) and the organic phase was concentrated to remove the THF. The residue was diluted with EtOAc (4 L), washed with IN aq HC1 (2 X 2 L), sat. aq. NaHC03 (2 L) and sat. aq. Na2C03 (2 L) and brine (1.5 L). After drying over Na2S04, the organic solvent was removed under reduce pressure to afford (R)-benzyl (l-((2,2-dimethoxyethyl)amino)-3-methyl-l-oxobutan-2- yl)carbamate as a white solid (670 g, yield 99.5%), which was used for next step without further purification.LC-MS m/z 360.9 [M+Na]+ XH NMR (CD3OD 300MHz): δ 7.35-7.30 (m, 5H), 5.08 (s, 2H), 4.45-4.35 (m, 1H), 3.95-3.85 (m, 1H), 3.34-3.25 (m, 8H), 2.10-1.90 (m, 1H), 0.94-0.91 (m, 6H).
References:
WO2013/138565,2013,A1 Location in patent:Page/Page column 38; 39