
2H-1-Benzopyran-2-one, 7-hydroxy-5-Methoxy-4-Methyl-3-(4-Methyl-1-piperazinyl)- synthesis
- Product Name:2H-1-Benzopyran-2-one, 7-hydroxy-5-Methoxy-4-Methyl-3-(4-Methyl-1-piperazinyl)-
- CAS Number:1456807-80-9
- Molecular formula:C16H20N2O4
- Molecular Weight:304.34
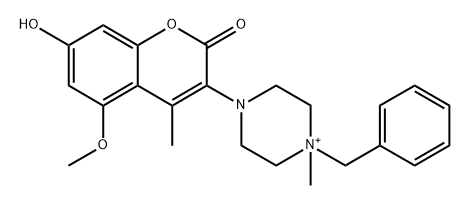
1456807-86-5
2 suppliers
inquiry

1456807-80-9
10 suppliers
inquiry
Yield: 91%
Reaction Conditions:
with palladium on activated charcoal;ammonium formate in methanol for 0.666667 h;Reflux;
Steps:
4.1.7 Synthesis of 7-hydroxy-5-methoxy-4-methyl-3-(4-methylpiperazin-1-yl)-2H-chromen-2-one (20)
General procedure: The intermediate compound 1 was prepared in a similar manner to the synthesis of compound 5, substituting piperazine with 1-methylpiperazine. To a solution of 1 (20mmol) in pyridine (20mL) at 0°C was added TsCl (50mmol) slowly. The mixture was stirred at room temperature for 2h. When the reaction was completed as monitored by LC-MS, the pyridine was evaporated in vacuo and water (50mL) was added to the residue followed by extraction with ethyl acetate (3×30mL). The organic layers were combined, dried over anhydrous MgSO4, and evaporated in vacuo. Intermediate 23 was obtained as yellow powder after chromatography by silica gel eluting with DCM-CH3OH (20:1, v/v). Yield 89%; 1H NMR (400MHz, DMSO-d6) δ 7.75 (d, J=8.3Hz, 2H), 7.62 (d, J=8.3Hz, 2H), 7.48 (d, J=8.2Hz, 2H), 7.44 (d, J=8.2Hz, 2H), 7.08 (d, J=2.4Hz, 1H), 6.69 (d, J=2.4Hz, 1H), 2.88 (s, 4H), 2.40 (s, 6H), 2.34 (s, 4H), 2.32 (s, 3H), 2.17 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ 156.2, 151.6, 147.9, 146.7, 146.4, 145.6, 139.1, 135.6, 130.6, 130.6, 130.4, 128.3, 128.2, 114.9, 112.6, 109.2, 55.1, 49.1, 46.0, 21.2, 21.1, 17.2. (0033) To a solution of compound 23 (15mmol) in acetone (100mL) was added K2CO3 (22.5mmol) and BnBr (15mmol) with stirring at room temperature. When the reaction was completed as monitored by LC-MS, the insoluble substrate was filtered off and the filtrate was evaporated in vacuo. The intermediate 24 was obtained as light-yellow powder after chromatography by silica gel eluting with DCM-CH3OH (20:1, v/v). Yield 87%; 1H NMR (300MHz, DMSO-d6) δ 7.78-7.71 (m, 4H), 7.65-7.58 (m, 2H), 7.58-7.47 (m, 7H), 7.20 (d, J=2.4, 1H), 6.69 (d, J=2.4, 1H), 4.74 (s, 2H), 3.67 (t, J=10.8, 2H), 3.52 (t, J=12.5, 2H), 3.41-3.37 (m, 2H), 3.05 (s, 3H), 3.02 (brs, 2H), 2.48 (s, 3H), 2.45 (s, 3H), 2.44 (s, 3H). (0034) Intermediate 24 (12mmol) was dissolved THF (100mL). The mixture was added with TBAF (12mmol) in ice bath and kept at 0°C for 4h with stirring. Then the solvent was evaporated in vacuo and the intermediate 25 was obtained as yellow powder after chromatography by silica gel eluting with DCM-CH3OH (15:1, v/v). Yield 87%; 1H NMR (300MHz, DMSO-d6) δ 7.74 (d, J=8.3, 2H), 7.62-7.53 (m, 5H), 7.42 (d, J=8.1, 2H), 6.17 (d, J=1.5, 1H), 6.01 (d, J=1.9, 1H), 4.71 (s, 2H), 3.65 (brs, 4H), 3.39 (brs, 4H), 2.99 (s, 3H), 2.72 (s, 3H), 2.37 (s, 3H). (0035) To a solution of 25 (9mmol) in acetone (70mL) was added K2CO3 (15mmol) and Me2SO4 (15mmol) successively with stirring at room temperature. 5 min later, the mixture was refluxed for 2h. When the reaction was completed as monitored by LC-MS, the insoluble substrate was filtered off and the filtrate was evaporated in vacuo. The intermediate 26 was obtained as yellow powder after chromatography by silica gel eluting with DCM-CH3OH (15:1, v/v). Yield 82%; 1H NMR (300MHz, DMSO-d6) δ 7.82 (d, J=8.3, 2H), 7.65-7.46 (m, 7H), 6.68 (d, J=2.3, 1H), 6.62 (d, J=2.3, 1H), 4.74 (s, 2H), 3.76 (s, 3H), 3.67-3.37 (m, 8H), 3.00 (s, 3H), 2.60 (s, 3H), 2.42 (s, 3H). (0036) TBAF (12mmol) was added to the solution of 26 (6mmol) in THF (30mL) and the mixture was stirred at room temperature. When the reaction was completed as monitored by LC-MS, the solvent was evaporated in vacuo. The intermediate 27 was obtained as yellow powder after chromatography by silica gel eluting with DCM-CH3OH (15:1, v/v). Yield 85%; 1H NMR (400MHz, DMSO-d6) δ 7.59-7.52 (m, 5H), 6.02 (s, 1H), 5.85 (s, 1H), 4.67 (s, 2H), 3.71 (s, 3H), 3.68 (brs, 4H), 3.32-3.30 (m, 2H), 2.97 (s, 3H), 2.74-2.72 (m, 2H), 2.56 (s, 3H). (0037) To the solution of 27 (4mmol) in CH3OH (20mL) was added Pd/C (8mmol) and HCOONH4 (20mmol). The reaction mixture was refluxed for 40min. After filtration, the filtrate was concentrated in vacuo and the final product 20 was obtained as pale-yellow powder after chromatography by silica gel eluting with DCM-CH3OH (20:1, v/v). Yield 91%; mp 199-201°C. 1H NMR (300Hz, DMSO-d6) δ 10.428 (s, 1H), 6.347 (d, J=2.1Hz, 1H), 6.266 (d, J=1.8Hz, 1H), 3.812 (s, 3H), 3.60-2.20 (m, 8H), 2.586 (s, 3H), 2.204 (s, 3H). 13C NMR (100MHz, DMSO-d6) δ 160.5, 159.2, 157.6, 154.2, 148.6, 129.2, 103.1, 96.2, 95.0, 55.9, 55.3, 48.9, 46.1, 17.0. HRMS calcd for C16H21N2O4 (M+H+) 305.1501; found. 305.1503.
References:
Sun, Mingna;Hu, Jinfeng;Song, Xiuyun;Wu, Donghui;Kong, Linglei;Sun, Yupeng;Wang, Dongmei;Wang, Yan;Chen, Naihong;Liu, Gang [European Journal of Medicinal Chemistry,2013,vol. 67,p. 39 - 53]
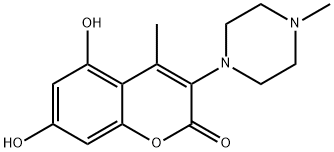
1209261-56-2
13 suppliers
$660.33/5g
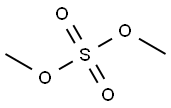
77-78-1
300 suppliers
$19.69/1ml
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1456807-80-9
10 suppliers
inquiry
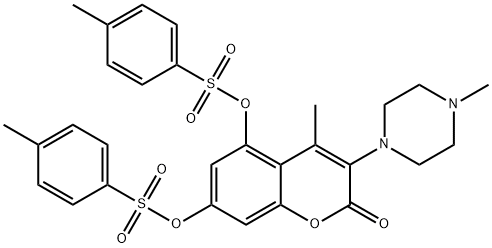
1456807-82-1
3 suppliers
inquiry

1456807-80-9
10 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1456807-80-9
10 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1456807-80-9
10 suppliers
inquiry