
6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid synthesis
- Product Name:6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid
- CAS Number:145739-56-6
- Molecular formula:C19H18N2O4S
- Molecular Weight:370.42
Yield:145739-56-6 78.4%
Reaction Conditions:
Stage #1: 2-(2-chloroacetyl)-6-pyridine carboxylic acid;3,4-diethoxybenzthioamide in 1,2-dimethoxyethane;water; for 2 h;Heating / reflux;
Stage #2: with potassium hydroxide in water;Product distribution / selectivity;
Steps:
1.6
20 g of 2- (2-chloroacetyl) -6-pyridine carboxylic acid and 22.6 g of 3, 4-diethoxythiobenzamide were dissolved in a solution consisting of 100 ml of water and 200 ml of dimethoxyethane . The obtained solution was heated to reflux for 2 hours while stirring, and the reaction solution was then cooled to 5°C or lower, so as to obtain a yellow-brown precipitate by filtration. [0169]Subsequently, the above precipitated crystal was dissolved in a solution formed by dissolving 6.18 g
References:
WO2007/119496,2007,A1 Location in patent:Page/Page column 65-68
![2-Pyridinecarboxylic acid, 6-[2-(3,4-diethoxyphenyl)-4-thiazolyl]-, methyl ester](/CAS/20180703/GIF/160322-91-8.gif)
160322-91-8
4 suppliers
inquiry
![6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid](/CAS/GIF/145739-56-6.gif)
145739-56-6
25 suppliers
inquiry
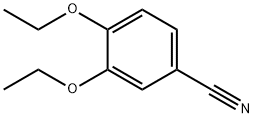
60758-87-4
46 suppliers
inquiry
![6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid](/CAS/GIF/145739-56-6.gif)
145739-56-6
25 suppliers
inquiry
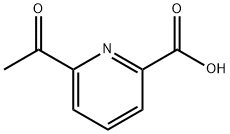
122637-39-2
52 suppliers
$56.00/100mg
![6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid](/CAS/GIF/145739-56-6.gif)
145739-56-6
25 suppliers
inquiry
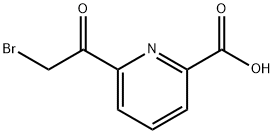
160322-90-7
1 suppliers
inquiry
![6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid](/CAS/GIF/145739-56-6.gif)
145739-56-6
25 suppliers
inquiry

