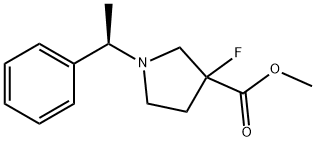
methyl 3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidine-3-carboxylate synthesis
- Product Name:methyl 3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidine-3-carboxylate
- CAS Number:1461698-11-2
- Molecular formula:C14H18FNO2
- Molecular Weight:251.3
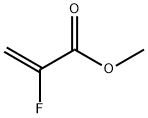
2343-89-7
244 suppliers
$6.00/1g
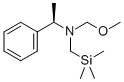
133407-38-2
62 suppliers
$45.00/50mg
![methyl 3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidine-3-carboxylate](/CAS/20210111/GIF/1461698-11-2.gif)
1461698-11-2
4 suppliers
inquiry
Yield:1461698-11-2 92%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0 - 20; for 5 h;Inert atmosphere;
Steps:
4.5. Methyl 3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidine-3-carboxylate (13)
A solution of TFA (1.8 g, 0.015 mol, 0.1 equiv) in dry CH2Cl2(150 mL) was slowly added to a stirred solution of (R)-12 (40.0 g,0.159 mol, 1.03 equiv) and methyl 2-fluoroacrylate 3 (16.0 g,0.153 mol, 1 equiv) in dry CH2Cl2 (250 mL) at 0 C. (CAUTION:exothermic reaction) The reaction mixture was stirred for an additional5 h at room temperature. The reaction mixture was neutralizedby adding a saturated aqueous solution of NaHCO3. The organic phase was washed with saturated solution of NaCl(2503 mL), separated and dried over anhydrous Na2SO4. Thesolvent was removed under reduced pressure on a rotary evaporator.The residue was purified by column chromatography usinghexane/EtOAc3/1 mixture as eluent to give the pure product 13(35.3 g, 0.14 mol, 92% yield) as a colorless oil. Rf0.5.1HNMR(500MHz; CDCl3,Me4Si; 2 diastereoisomers are present)d: 1.37e1.40 (m, 3H, CHCH3), 2.12e2.28 (m, 1H), 2.33e2.51 (m, 2H),2.68e2.79 (m,1H), 2.83e3.02 (m, 1H), 3.05e3.12 (m, 1H), 3.28e3.37(m, 1H), 3.76e3.79 (m, 3H, OCH3), 7.20e7.33 (m, 5H, C6H5).13C NMR (125 MHz; CDCl3; Me4Si; 2 diastereoisomers arepresent), d: 23.0 (CH3), 36.9, 51.6, 52.8, 62.6, 65.0, 99.9 (m,JCF193 Hz, CF), 127.0 (s, C6H5), 127.2 (s, C6H5), 128.4 (s, C6H5), 144.7(s, C6H5), 171.4 (s, C]O).19F NMR (477 MHz; CDCl3; C6F6; 2 diastereoisomers are present)d: 148.2 (147.6) (m, J26 Hz).Anal. Calcd for C14H18FNO2: C, 66.91; H, 7.22; N, 5.57. Found: C,66.84; H, 7.35; N, 5.42.LCeMS: 252 (M).[a]D20 17.03 (c 0.702 g/100 mL, MeOH).
References:
Yarmolchuk, Vladimir S.;Mykhalchuk, Vladimir L.;Mykhailiuk, Pavel K. [Tetrahedron,2014,vol. 70,# 18,p. 3011 - 3017]