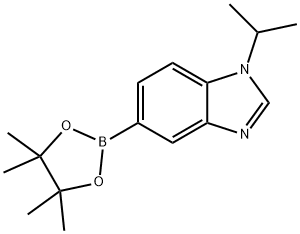
1H-Benzimidazole, 1-(1-methylethyl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- synthesis
- Product Name:1H-Benzimidazole, 1-(1-methylethyl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
- CAS Number:1463053-97-5
- Molecular formula:C16H23BN2O2
- Molecular Weight:286.18
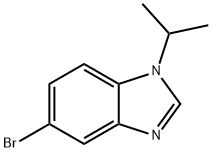
1200114-01-7
21 suppliers
$75.00/50mg

73183-34-3
566 suppliers
$6.00/5g

1463053-97-5
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 100; for 4 h;Inert atmosphere;Sealed tube;
Steps:
17 PREPARATION xl7: l-isopropyl-5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)- lH-benzo[d]imidazole
PREPARATION xl7: l-isopropyl-5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)- lH-benzo[d]imidazole [0222] 5-Bromo-l-isopropyl-lH-benzo[d]imidazole (500 mg, 2.091 mmol), potassium acetate (694 mg, 7.07 mmol) and 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (719 mg, 2.83 mmol) were mixed in dioxane and degassed with N2 for 20 minutes. PdCl2(dppf)CH2Cl2 adduct (193 mg, 0.236 mmol) was added in one portion and the reaction mixture was sealed and heated to 100°C for 4 hours. The mixture, which contained the title compound, was used without further purification.
References:
WO2013/148603,2013,A1 Location in patent:Paragraph 0221-0222