
Acetamide, N-[2-(4-bromophenyl)-2-hydroxyethyl]-2-chloro- synthesis
- Product Name:Acetamide, N-[2-(4-bromophenyl)-2-hydroxyethyl]-2-chloro-
- CAS Number:1467061-25-1
- Molecular formula:C10H11BrClNO2
- Molecular Weight:292.56

41147-82-4
31 suppliers
$45.00/50mg

79-04-9
352 suppliers
$12.00/5g
![Acetamide, N-[2-(4-bromophenyl)-2-hydroxyethyl]-2-chloro-](/CAS/20210111/GIF/1467061-25-1.gif)
1467061-25-1
0 suppliers
inquiry
Yield:1467061-25-1 76%
Reaction Conditions:
with sodium hydroxide in dichloromethane;water at 0 - 20; for 6 h;
Steps:
79.1 N-(2-(4-bromophenyl)-2-hydroxyethyl)-2-chloroacetamide
To a solution of compound 2-amino-1-(4-bromophenyl)ethanol (2.0 g, 10 mmol) in CH2Cl2 (40 mL) and water (40 mL) was added NaOH (0.48 g, 12 mmol) and chloroacetyl chloride (1.7 g, 15 mmol) at 0° C.
After addition, the mixture was allowed to warm to room temperature and stirred for 6 hours.
The layers were separated.
The organic was washed with 3% HCl and saturated NaHCO3, dried over sodium sulfate, and concentrated to afford the title compound (2.0 g, 76% yield) as a yellow solid. 1H NMR: (400 MHz, CDCl3): δ 7.45 (d, 2H), 7.19 (d, 2H), 6.98 (br. s, 1H), 4.80 (m, 1H), 4.01 (s, 2H), 3.68 (m, 1H), 3.30 (m, 1H), 2.82 (br. s, 1H):
References:
US2013/267493,2013,A1 Location in patent:Paragraph 1062

7644-04-4
41 suppliers
inquiry
![Acetamide, N-[2-(4-bromophenyl)-2-hydroxyethyl]-2-chloro-](/CAS/20210111/GIF/1467061-25-1.gif)
1467061-25-1
0 suppliers
inquiry
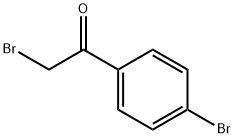
99-73-0
228 suppliers
$10.00/1g
![Acetamide, N-[2-(4-bromophenyl)-2-hydroxyethyl]-2-chloro-](/CAS/20210111/GIF/1467061-25-1.gif)
1467061-25-1
0 suppliers
inquiry