
2-(4-(5,5-Dimethyl-1,3,2-dioxaborinan-2-yl)phenyl)-1,1,1-trifluoropropan-2-ol synthesis
- Product Name:2-(4-(5,5-Dimethyl-1,3,2-dioxaborinan-2-yl)phenyl)-1,1,1-trifluoropropan-2-ol
- CAS Number:1467061-57-9
- Molecular formula:C14H18BF3O3
- Molecular Weight:302.1
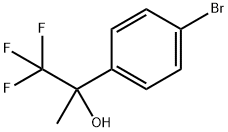
122243-28-1
30 suppliers
inquiry
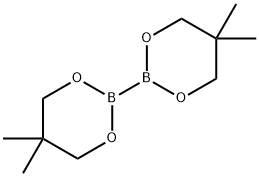
201733-56-4
258 suppliers
$5.00/1g

1467061-57-9
18 suppliers
inquiry
Yield:1467061-57-9 74%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 110; for 1 h;Inert atmosphere;Microwave irradiation;
Steps:
108.1 2-(4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)phenyl)-1,1,1-trifluoropropan-2-ol
A mixture of 2-(4-bromophenyl)-1,1,1-trifluoropropan-2-ol (300 mg, 1.12 mmol), 5,5,5',5'-tetramethyl-2,2'-bi-1,3,2-dioxaborinane (420 mg, 1.23 mmol), potassium acetate (500 mg, 5.10 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (90 mg, 0.11 mmol) was purged with N2, suspended in degassed 1,4-dioxane (2.0 mL) and subjected to microwave irradiation at 110° C. for 60 minutes.
The cooled reaction mixture was diluted with water (15 mL) and extracted with EtOAc (2*20 mL).
The combined organic layers were washed with water and brine, dried over MgSO4 and concentrated in vacuo.
The resulting black oil was purified by flash chromatography (0-67% EtOAc/heptane) to afford the title compound (249 mg, 74% yield) as a pale yellow oil. GC/MS, M=302 at 3.58 min. 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J=8.39 Hz, 2H), 7.54 (d, J=8.00 Hz, 2H), 3.76 (5, 4H), 1.77 (5, 3H), 1.01 (5, 6H).
References:
US2013/267493,2013,A1 Location in patent:Paragraph 1164