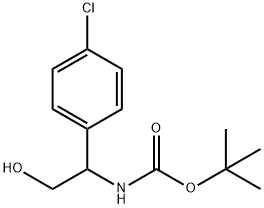
tert-butyl 1-(4-chlorophenyl)-2-hydroxyethylcarbaMate synthesis
- Product Name:tert-butyl 1-(4-chlorophenyl)-2-hydroxyethylcarbaMate
- CAS Number:147353-95-5
- Molecular formula:C13H18ClNO3
- Molecular Weight:271.74
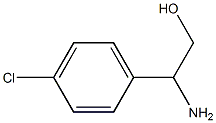
179811-64-4
35 suppliers
$119.17/0.25g

24424-99-5
825 suppliers
$13.50/25G

147353-95-5
28 suppliers
$114.00/250mg
Yield:147353-95-5 88%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20;
Steps:
105.1
Step 1: (rac)-tert-butyl (1 -(4-chlorophenyl)-2-hydroxyethyl)carbamate:To a solution of (rac)-2-amino-2-(4-chlorophenyl)ethanol (2 g, 1 1 .65 mmol) (prepared as described WO2009/47563 A1 ) in THF (20 ml) was added Boc anhydride (2.84 ml, 12.24 mmol) followed by triethylamine (2.436 ml, 17.48 mmol) . The reaction mixture was then stirred at RT and maintained at this temperature overnight. The reaction mixture was concentrated in vacuo, diluted with EtOAc and 1 N HCI and extracted. The organic extracts were then washed with brine, dried (Na2S04) and concentrated in vacuo affording the title compound as a sticky solid (2.8 g, 88%). 1H NMR (500 MHz, CDCI3): 7.34 (2H, m), 7.27 (1 H, d), 5.35 (1 H, m), 4.75 (1 H, m), 3.75- 3.90 (2H, m), 1 .44 (9H, s).
References:
WO2012/69852,2012,A1 Location in patent:Page/Page column 137-138

24424-99-5
825 suppliers
$13.50/25G
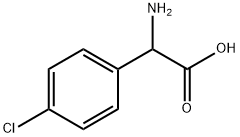
6212-33-5
308 suppliers
$6.00/5g

147353-95-5
28 suppliers
$114.00/250mg