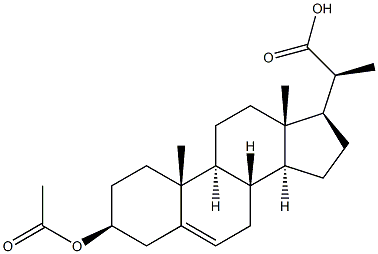
Pregn-5-ene-20-carboxylicacid, 3-(acetyloxy)-, (3b,20S)- synthesis
- Product Name:Pregn-5-ene-20-carboxylicacid, 3-(acetyloxy)-, (3b,20S)-
- CAS Number:1474-14-2
- Molecular formula:C24H36O4
- Molecular Weight:388.5403

10211-88-8
6 suppliers
inquiry

1474-14-2
2 suppliers
inquiry
Yield:1474-14-2 91%
Reaction Conditions:
with sodium chlorite;dipotassium hydrogenphosphate in tetrahydrofuran;water;tert-butyl alcohol at 0 - 20;
Steps:
18 [00386] Preparation of compound B7d:
[00386] Preparation of compound B7d: Cholest-5-en-3p-ol-22-al B7c (1.33 g, 3.57 mmol) was dissolved in t-butanol (75ml), tetrahydrofuran (dry) (15 ml) and 2-methyl-2- butene (13.22 ml, 125 mmol). The solution was stirred and cooled with an iced bath. A freshly prepared solution of sodium chlorite (0.355 g, 3.93 mmol) and potassium phosphate, monobasic, p. a. (0.534 g, 3.93 mmol) in demineralized water (45 ml) was slowly added to the solution over a period of 30 minutes and the mixture was stirred 2 hour at 0°C. The ice bath was removed, the temperature of the mixture was raised to room temperature, and stirred overnight. TLC [heptane (2): ethyl acetate(l)] showed partial conversion to a lower eluting product after vanillin staining. Extra sodium chlorite (0.355 g, 3.93 mmol) and potassium phosphate, monobasic, p. a. (0.534 g, 3.93 mmol) dissolved in water (45 ml) was added slowly to the reaction mixture and stirring was continued for 2h. TLC[heptane(2):ethyl acetate(l)] showed complete conversion to a lower eluting product after vanillin staining. The reaction mixture was poured into saturated aqueous ammonium chloride (250 ml) and extracted three times with dichloromethane (100 ml). The combined organic layers were dried over sodium sulfate, filtered and evaporated under reduced pressure. The residue was twice stripped with toluene (50 ml) followed by dichloromethane (50 ml). The white solid residue (2.26 g, 163%) was triturated in petroleum ether 40-60 (10 ml) for 0.5h. The white solid was filtered, washed twice with petroleum ether 40-60 (10 ml) and dried on air (leaving the vacuum pump on) for 0.5h to obtain B7d (1.27 g, 3.26 mmol, Yield=91%) as a white powder. B7d was used as such in the following experiment(s). 1HNMR (400 MHz, CDC13) 5(ppm): 10.31 (1H, bs), 5.37 (1H, brd), 4.65-4.56 (1H, m), 2.47-2.39 (1H, m), 2.36-2.26 (2H, m), 2.04 (3H, s), 2.01-1.92 (2H, m), 1.90-1.76 (3H, m), 1.24 (3H, d, J=6.8 Hz), 1.02 (3H, s), 0.71 (3H, s).
References:
WO2013/36835,2013,A1 Location in patent:Paragraph 00386
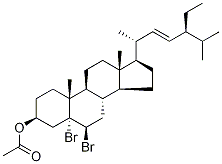
32212-73-0
7 suppliers
$155.00/50mg

1474-14-2
2 suppliers
inquiry
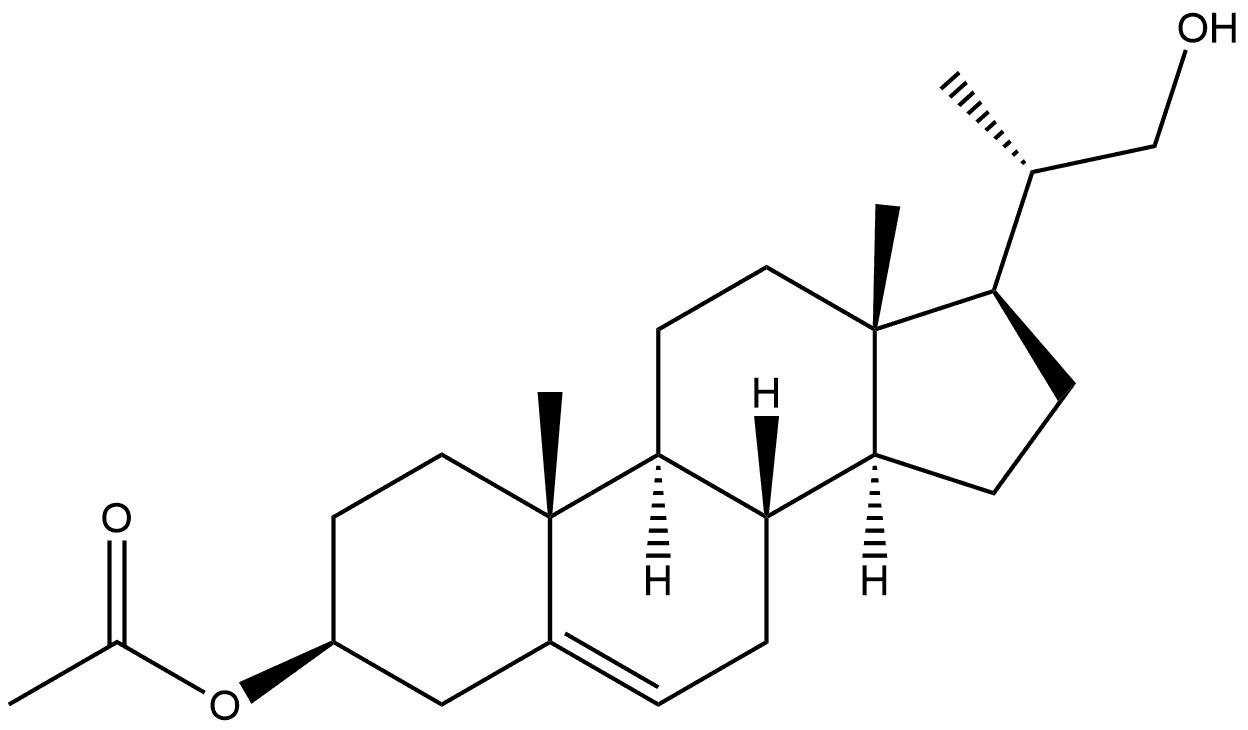
55509-37-0
0 suppliers
inquiry

1474-14-2
2 suppliers
inquiry

2458-53-9
2 suppliers
inquiry

1474-14-2
2 suppliers
inquiry
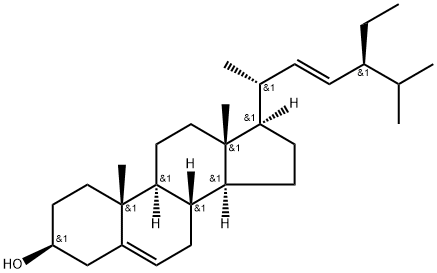
83-48-7
410 suppliers
$30.00/20mg

1474-14-2
2 suppliers
inquiry