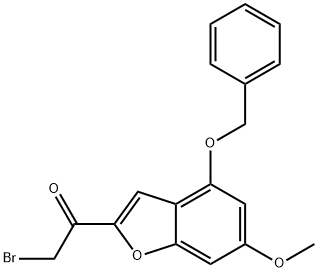
1-(4-(benzyloxy)-6-methoxybenzofuran-2-yl)-2-bromoethanone synthesis
- Product Name:1-(4-(benzyloxy)-6-methoxybenzofuran-2-yl)-2-bromoethanone
- CAS Number:1476847-52-5
- Molecular formula:C18H15BrO4
- Molecular Weight:375.21
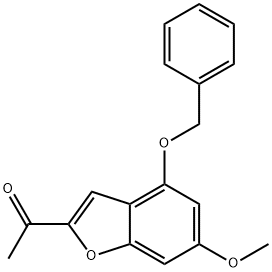
1476847-51-4
7 suppliers
inquiry

1476847-52-5
15 suppliers
inquiry
Yield:1476847-52-5 86%
Reaction Conditions:
Stage #1: 1-[4-(benzyloxy)-6-methoxy-1-benzofuran-2-yl]ethan-1-onewith lithium hexamethyldisilazane in tetrahydrofuran at -78; for 0.75 h;Inert atmosphere;
Stage #2: with chloro-trimethyl-silane in tetrahydrofuran at -78; for 0.333333 h;
Stage #3: with N-Bromosuccinimide;sodium hydrogencarbonate in tetrahydrofuran at 0; for 2 h;
Steps:
203.203D 1-(4-(Benzyloxy)-6-methoxybenzofuran-2-yl)-2-bromoethanone
A 250-mL, three-necked flask is equipped with a magnetic stirring bar and purged with a nitrogen atmosphere was charged with anhydrous tetrahydrofuran (25 mL) followed by 9.3 mL (9.3 mmol) of a 1M solution of lithium bis(trimethylsilyl)amide in tetrahydrofuran. The mixture was cooled to -78 °C and treated with a solution of 1-(4-(benzyloxy)-6-methoxybenzofuran-2-yl)ethanone (Example 203C, 2.40 g, 8.1 mmole) in tetrahydrofuran (20 mL) added dropwise over 10 min. The resulting mixture was then stirred at -78 °C for 45 min. Then chlorotrimethylsilane (1.18 mL, 9.31 mmol) was added dropwise over 5 min and the resulting solution was stirred at -78 °C for another 20 min. The cooling bath was then removed and the mixture is allowed to warm to room temperature over 30 min. The reaction mixture was then quenched by addition to a cold solution of ethyl acetate (200 mL), saturated sodium bicarbonate (30 mL) and ice. The organic phase was rapidly dried over anhydrous magnesium sulfate (magnetic stirring) and evaporated in vacuo to give the silyl enol ether as an oil which is co-evaporated with toluene (20 mL). The silyl enol ether was then dissolved in dry tetrahydrofuran (40 mL), cooled to -20 °C and treated with solid sodium bicarbonate (0.10 g) followed by N- bromosuccinimide (1.44 g, 8.1 mmol) added in small portions over 15 min. The reaction mixture was allowed to warm to 0 °C over 2h and then quenched by addition of ethyl acetate (300 mL) and saturated sodium bicarbonate. The organic phase was washed with brine, dried over anhydrous magnesium sulfate and evaporated to give an orange oil. Chromatography on silica gel (4 x 12 cm, elution toluene - ethyl acetate 0-5%) gave 2.62 g (86% yield) of the title bromomethylketone as a yellow solid. Recrystallization from ethyl acetate (10 mL) and hexane (20 mL) gave yellow prisms (2.30 g). LC (Method B): 1.977 min. HRMS(ESI) calcd for C18H16BrO4 [M+H]+ m/z 375.0226, found 375.0277. 1H NMR (CDCl3, 600 MHz) δ 3.84 (s, 3H), 4.33 (s, 2H), 5.14 (s, 2H), 6.38 (d, J = 1.76 Hz, 1H), 6.64 (broad s, 1H), 7.35 (broad t, 1H), 7.40 (broad t, 2H), 7.44 (broad d, 2H), 7.70 (s, 1H).
References:
WO2013/163244,2013,A1 Location in patent:Paragraph 00229
1476847-49-0
0 suppliers
inquiry

1476847-52-5
15 suppliers
inquiry
532394-23-3
12 suppliers
inquiry

1476847-52-5
15 suppliers
inquiry
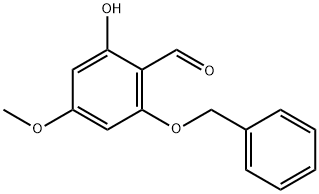
1476847-50-3
12 suppliers
inquiry

1476847-52-5
15 suppliers
inquiry

100-39-0
422 suppliers
$10.00/10g

1476847-52-5
15 suppliers
inquiry