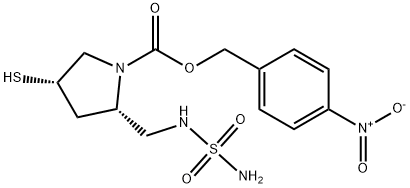
(2R,4S)-4-nitrobenzyl 4-Mercapto-2-((sulfaMoylaMino)Methyl)pyrrolidine-1-carboxylate synthesis
- Product Name:(2R,4S)-4-nitrobenzyl 4-Mercapto-2-((sulfaMoylaMino)Methyl)pyrrolidine-1-carboxylate
- CAS Number:148017-03-2
- Molecular formula:C13H18N4O6S2
- Molecular Weight:390.44
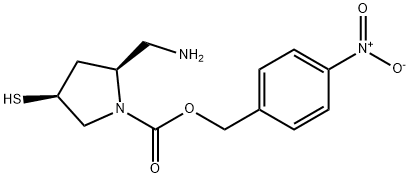
148017-06-5
0 suppliers
inquiry

148017-03-2
18 suppliers
inquiry
Yield:148017-03-2 75%
Reaction Conditions:
Stage #1: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-aminomethyl-4-mercaptopyrrolidinewith chloro-trimethyl-silane;triethylamine in dichloromethane at -75; for 2 h;Inert atmosphere;
Stage #2: with sulphamoyl chloride;triethylamine in dichloromethane; for 1 h;Temperature;
Steps:
1-3
Compound VI (3.00g, 0.0096mol) was dissolved in dichloromethane, and the temperature was reduced to -75°C under nitrogen protection.Add triethylamine (1.30g, 0.0128mol), add dropwise trimethylchlorosilane (1.25g, 0.0115mol),After dripping, stir for 2 hours; add triethylamine (1.46g, 0.0144mol),Add a dichloromethane solution of aminosulfonyl chloride (1.34g, 0.0116mol) dropwise, and stir for 1 hour;Add a mixture of dilute hydrochloric acid and methanol, stir for 40 minutes, and wash with brine to obtain an organic phase.The organic phase was concentrated to dryness, and ethyl acetate (20ml) was added for crystallization to obtain compound I (3.37g, purity 98%, molar yield 75%);
References:
CN111960984,2020,A Location in patent:Paragraph 0051-0053; 0062-0063; 0064-0066; 0070; 0071-0073
![1,2-Pyrrolidinedicarboxylic acid, 4-[(Methylsulfonyl)oxy]-, 2-Methyl 1-[(4-nitrophenyl)Methyl] ester, (2S,4R)-](/CAS/GIF/138324-82-0.gif)
138324-82-0
16 suppliers
inquiry

148017-03-2
18 suppliers
inquiry
![1,2-Pyrrolidinedicarboxylic acid, 4-hydroxy-, 2-methyl 1-[(4-nitrophenyl)methyl] ester, (2S,4R)-](/CAS/20210305/GIF/101803-29-6.gif)
101803-29-6
0 suppliers
inquiry

148017-03-2
18 suppliers
inquiry