
(2E)-1-(2,4-dichlorophenyl)-3-(4-ethoxyphenyl)prop-2-en-1-one synthesis
- Product Name:(2E)-1-(2,4-dichlorophenyl)-3-(4-ethoxyphenyl)prop-2-en-1-one
- CAS Number:1486549-12-5
- Molecular formula:C17H14Cl2O2
- Molecular Weight:321.2
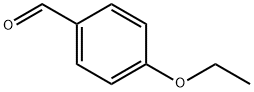
10031-82-0
271 suppliers
$6.00/5g

2234-16-4
427 suppliers
$6.00/25g

1486549-12-5
4 suppliers
inquiry
Yield:1486549-12-5 79%
Reaction Conditions:
with potassium hydroxide in ethanol; for 3 h;Reflux;Solvent;Temperature;Time;
Steps:
Method A.
General procedure: The 4% ethanolic solution of KOH (15 mL) was added to a stirred solution of 20,40-dichloro-acetophenone (0.5 g, 2.65 mmol, 1 eq) and aromatic aldehyde (2.65 mmol, 1 eq) in EtOH (25 mL) and resulting reaction mixture was refluxed for 3 h. The reaction was quenched with diluted aqueous. HCl, EtOH evaporated under a stream of N2, and resulting reaction mixture was partitioned between H2O (25 mL) and CH2Cl2 (350 mL). The organic extract was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to afford saturated solution that upon standing yielded crystals of enone (4a-e) in good yield (75-92%).
References:
Sultan, Aeysha;Raza, Abdul Rauf [Synthetic Communications,2014,vol. 44,# 3,p. 417 - 423]