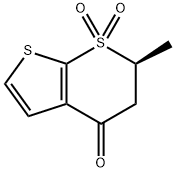
4H-Thieno[2,3-b]thiopyran-4-one,5,6-dihydro-6-methyl-, 7,7-dioxide, (6S) synthesis
- Product Name:4H-Thieno[2,3-b]thiopyran-4-one,5,6-dihydro-6-methyl-, 7,7-dioxide, (6S)
- CAS Number:148719-91-9
- Molecular formula:C8H8O3S2
- Molecular Weight:216.28
![(S)-6-Methyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-one](/CAS/GIF/147086-79-1.gif)
147086-79-1
100 suppliers
$310.00/250mg
![4H-Thieno[2,3-b]thiopyran-4-one,5,6-dihydro-6-methyl-, 7,7-dioxide, (6S)](/CAS/GIF/148719-91-9.gif)
148719-91-9
54 suppliers
$68.00/1 g
Yield: 80%
Reaction Conditions:
with sodium tungstate (VI) dihydrate;dihydrogen peroxide in ethyl acetate;toluene at 0 - 10;Product distribution / selectivity;
Steps:
4 obtaining (6S),-5,6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one-7,7-dioxide
EXAMPLE 4 obtaining (6S),-5,6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one-7,7-dioxide 10.22 g (0.055 moles) of (6S)-5.6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one were dissolved in 30 mL of toluene and 46 mL of ethyl acetate; it then was cooled to a temperature of 0-5°C and 2.65 g (0.008 moles) of Na2WO4·2H2O, 1.5 mL of water were loaded. Then, 19.8 mL (0.19 moles) of 30% H2O2 were added, keeping the temperature below 10°C and it was stirred until finishing the starting product. The phases were decanted and the organic phase was washed with an aqueous solution of NaCl. The aqueous phase was extracted with ethyl acetate (AcOEt) and the obtained organic phases were pooled together. The organic phase was distilled to a residue, 13 mL of methanol were added and the suspension was cooled at a temperature of 0-5°C, the obtained solid was filtered and dried, 8.5 g of a white solid being obtained, which corresponds to the compound of the title with an 80% yield. Mp: 97.5-98.2°C 13C NMR (CDCl3) δ: 187, 147, 141, 131, 127, 58, 45, 12 1H NMR (CDCl3) δ: 7.58 (1H, d), 7.46 (1H, d), 3.84 (1H, m), 3.18 (2H, m), 1.55 (3H, d) α D: -7.04 c: 1 in CH2Cl2
References:
Ragactives, S.L. EP2128161, 2009, A1 Location in patent:Page/Page column 11

133359-80-5
57 suppliers
inquiry
![4H-Thieno[2,3-b]thiopyran-4-one,5,6-dihydro-6-methyl-, 7,7-dioxide, (6S)](/CAS/GIF/148719-91-9.gif)
148719-91-9
54 suppliers
$68.00/1 g