
Methyl 1-(4-Methoxybenzyl)-5-oxopyrrolidine-3-carboxylate synthesis
- Product Name:Methyl 1-(4-Methoxybenzyl)-5-oxopyrrolidine-3-carboxylate
- CAS Number:149505-71-5
- Molecular formula:C14H17NO4
- Molecular Weight:263.29
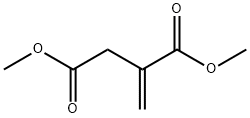
617-52-7
208 suppliers
$13.00/5g
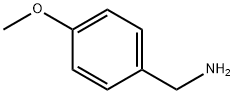
2393-23-9
463 suppliers
$20.00/25g

149505-71-5
29 suppliers
$45.00/50mg
Yield:149505-71-5 91%
Reaction Conditions:
in methanol at 20; for 2 h;Reflux;
Steps:
Methyl 1-(4-methoxybenzyl)-5-oxopyrrolidine-3-carboxylate (4a)
A mixture of dimethyl itaconate (2.37 g; 15 mmol), 4-methoxy-benzylamine (2.10 g; 15 mmol) and 1.5 mL of absolute methanol was prepared and stirred at room temperature overnight. The reaction mixture was refluxed for 2 h, the solvent was removed, and the residue was treated with water (30 mL). The solution was extracted with ethyl acetate (3 x 30 mL). The combined organic layers were washed with brine (20 mL) and dried over anhydrous sodium sulfate. The solvent was removed to give compound 4a as yellow oil (3.95 g; 91% yield). 1H NMR (CDCl3): δ 2.73 (t, J = 8.7 Hz, 2H), 3.16-3.24 (m, 1H), 3.43 (d, J = 7.7 Hz, 2H), 3.69 (s, 3H), 3.79 (s, 3H), 4.39 (dd, J = 20.3, 11.6 Hz, 2H), 6.84 (d, J = 8.6 Hz, 2H), 7.15 (d, J = 8.6 Hz, 2H).
References:
Dou, Dengfeng;He, Guijia;Mandadapu, Sivakoteswara Rao;Aravapalli, Sridhar;Kim, Yunjeong;Chang, Kyeong-Ok;Groutas, William C. [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 1,p. 377 - 379] Location in patent:supporting information; experimental part
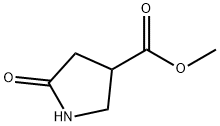
35309-35-4
139 suppliers
$20.00/1g
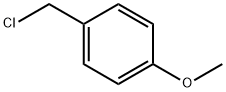
824-94-2
444 suppliers
$24.19/5G

149505-71-5
29 suppliers
$45.00/50mg

2393-23-9
463 suppliers
$20.00/25g

149505-71-5
29 suppliers
$45.00/50mg