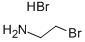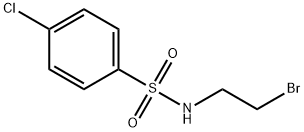
N1-(2-BROMOETHYL)-4-CHLOROBENZENE-1-SULFONAMIDE, TECH synthesis
- Product Name:N1-(2-BROMOETHYL)-4-CHLOROBENZENE-1-SULFONAMIDE, TECH
- CAS Number:151389-59-2
- Molecular formula:C8H9BrClNO2S
- Molecular Weight:298.58
Yield:151389-59-2 99%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 0;
Steps:
24 Preparation 24
N-(2-Bromoethyl)-4-chlorobenzenesulfonamide
Preparation 24
N-(2-Bromoethyl)-4-chlorobenzenesulfonamide
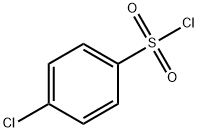
To a stirred mixture of 4-chlorobenzenesulfonyl chloride (5.0 g, 23.7 mmol) and 2-bromoethylamine hydrobromide (5.4 g, 26.3 mmol) in anhydrous methylene chloride (50 mL) at 0° C. was slowly added N,N-diisopropylethyl amine (8.6 mL, 52.1 mmol) and the mixture was stirred at 0° C. for 1 hour.
The reaction mixture was washed sequentially with water, 2N aqueous hydrochloric acid, saturated aqueous sodium carbonate, and saturated aqueous sodium chloride.
The organic layer was dried (anhydrous sodium sulfate), filtered, and concentrated in vacuo to afford a white solid (7 g, 99%).
1H NMR (300 MHz, CDCl3) δ 7.82 (d, 2H, J=8.7 Hz), 7.52 (d, 2H, J=8.7 Hz), 4.96 (s, 1H), 3.50-3.30 (m, 4H).
References:
US2013/303524,2013,A1 Location in patent:Paragraph 0285-0286