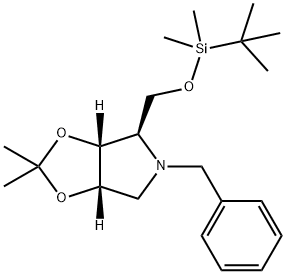
4H-1,3-Dioxolo[4,5-c]pyrrole, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]tetrahydro-2,2-dimethyl-5-(phenylmethyl)-, (3aR,4R,6aS)- synthesis
- Product Name:4H-1,3-Dioxolo[4,5-c]pyrrole, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]tetrahydro-2,2-dimethyl-5-(phenylmethyl)-, (3aR,4R,6aS)-
- CAS Number:153172-30-6
- Molecular formula:C21H35NO3Si
- Molecular Weight:377.59
Yield:153172-30-6 98%
Reaction Conditions:
with 1H-imidazole;dmap in dichloromethane at 20; for 10 h;Inert atmosphere;
Steps:
4.1. 1-Amino-1,4-anhydro-N-benzyl-5-O-tert-butyldimethylsilyl-1-deoxy-2,3-O-isopropylidene-d-ribitol (7)
To a stirred solution of 6refPreviewPlaceHolder37 (150 mg, 0.57 mmol) in anhydrous CH2Cl2 (5 mL) at rt under Ar atmosphere were added DMAP (7 mg, 0.05 mmol) and imidazole (93 mg, 1.36 mmol) followed by TBDMSCl (103 mg, 0.68 mmol). The mixture was then stirred for 10 h and partitioned (CH2Cl2//·NaHCO3/H2O). The organic layer was washed (brine), dried (MgSO4) and evaporated. The residue was purified by column chromatography (15% EtOAc/hexane) to give 7refPreviewPlaceHolder50 (204 mg, 98%) as a colorless oil. 1H NMR δ -0.13 (s, 3, CH3), 0.00 (s, 3, CH3), 0.83 (s, 9, t-Bu), 1.26 (s, 3, CH3), 1.49 (s, 3, CH3), 2.65 (dd, J = 2.7, 10.3 Hz, 1, H1), 2.94 (‘q’, J = , 2.2 Hz, 1, H4), 3.04 (dd, J = 5.5, 10.3 Hz, 1, H1'), 3.57 (dd, J = 4.1, 10.6 Hz, 1, H5), 3.64 (d, J = 13.4 Hz, 1, Bn), 3.71 (dd, J = 4.3, 10.6 Hz, 1, H5'), 3.94 (d, J = 13.4 Hz, 1, Bn), 4.49 (dd, J = 2.0, 6.5 Hz, 1, H3), 4.58 (‘dt’, J = 2.7, 6.2 Hz, 1, H2), 7.13-7.23 (m, 5, Bn); 13C NMR δ -5.5, (CH3), -5.4, (CH3), 18.2 (t-Bu), 25.2 (CMe2), 25.7 (CH3), 25.9 (CH3), 27.2 (CMe2), 56.9 (Bn), 59.3 (C1), 63.2 (C5), 68.9 (C4), 79.4 (C2), 83.3 (C3), 111.9 (CMe2), 128.2, 128.5, 126.8, 139.4 (Bn); MS (APCI) m/z 378 (100, MH+).
References:
Malladi, Venkata L.A.;Sobczak, Adam J.;Meyer, Tiffany M.;Pei, Dehua;Wnuk, Stanislaw F. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 18,p. 5507 - 5519] Location in patent:experimental part
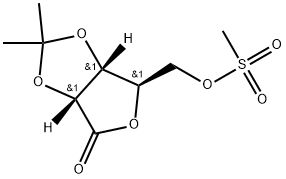
89747-54-6
2 suppliers
inquiry
![4H-1,3-Dioxolo[4,5-c]pyrrole, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]tetrahydro-2,2-dimethyl-5-(phenylmethyl)-, (3aR,4R,6aS)-](/CAS/20180703/GIF/153172-30-6.gif)
153172-30-6
10 suppliers
inquiry
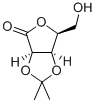
152006-17-2
75 suppliers
$95.00/500mg
![4H-1,3-Dioxolo[4,5-c]pyrrole, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]tetrahydro-2,2-dimethyl-5-(phenylmethyl)-, (3aR,4R,6aS)-](/CAS/20180703/GIF/153172-30-6.gif)
153172-30-6
10 suppliers
inquiry
![5-O-[(tert-Butyl)dimethylsilyl]-2,3-O-(1-methylethylidene)-L-lyxonic acid gamma-lactone](/CAS2/GIF/1044813-00-4.gif)
1044813-00-4
6 suppliers
inquiry
![4H-1,3-Dioxolo[4,5-c]pyrrole, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]tetrahydro-2,2-dimethyl-5-(phenylmethyl)-, (3aR,4R,6aS)-](/CAS/20180703/GIF/153172-30-6.gif)
153172-30-6
10 suppliers
inquiry
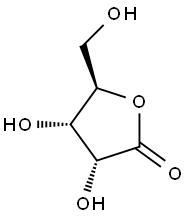
5336-08-3
195 suppliers
$10.00/100mg
![4H-1,3-Dioxolo[4,5-c]pyrrole, 4-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]tetrahydro-2,2-dimethyl-5-(phenylmethyl)-, (3aR,4R,6aS)-](/CAS/20180703/GIF/153172-30-6.gif)
153172-30-6
10 suppliers
inquiry

![[(3aR,4R,6aS)-5-benzyl-2,2-dimethyl-3a,4,6,6a-tetrahydro-[1,3]dioxolo[4,5-c]pyrrol-4-yl]methanol](/CAS/20180601/GIF/117858-84-1.gif)