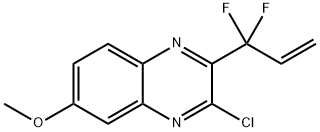
3-chloro-2-(1,1-difluoroallyl)-6-methoxyquinoxaline synthesis
- Product Name:3-chloro-2-(1,1-difluoroallyl)-6-methoxyquinoxaline
- CAS Number:1535210-94-6
- Molecular formula:C12H9ClF2N2O
- Molecular Weight:270.66

1535210-93-5
6 suppliers
inquiry

1535210-94-6
16 suppliers
inquiry
Yield:1535210-94-6 14.5 g
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-formamide at 50 - 74; for 2.5 h;Inert atmosphere;Temperature;Time;
Steps:
C4.B.6 Step 6. Preparation of Intermediate C4
Step 6. Preparation of Intermediate C4. Hydroxyquinoxaline (22.4 g, 88.8 mmol) was dissolved in DMF (45 mL) in a round-bottomed flask equipped with a temperature probe and N2 inlet. POCI3 (12.5 mL, 134 mmol, 1 .5 equiv) was added via syringe at a rate to keep the internal temperature below 50 °C. The dark red solution was then heated via heat block pre-heated to 75 °C (internal temperature ~ 74 °C). After 2.5 h, the reaction was then transferred via cannula to 370 mL of stirred H2O in a 3-neck flask equipped with temperature probe, overhead stirring, and vent to atmosphere. The rate of quench was controlled such that the internal temperature remained below 35 °C. Three additional portions of DMF (3 mL each) were used to ensure complete transfer. Once the internal temperature decreased to 30 °C, 3 M aqueous NaOH was added until a pH of -6-7 was obtained (160 mL total). The brown heterogeneous mixture was then cooled to an internal temperature of 15 °C and was filtered through an M- grade frit. The filter cake was washed with H2O (2 x 30 mL) and 3:1 H2O:MeCN (3 x 20 mL). The filter cake was suspended in CH2CI2 (200 mL), and the mixture was dried with anhydrous MgSO4 and filtered through a short pad of Celite. Concentration in vacuo provided 19 g of a dark red oil. This oil was dissolved in CH2CI2 (100 mL) and was slurried with silica gel (40 g) for 20 min. The slurry was filtered through a short pad of fresh silica gel (20 g), washing with 6 x 40 mL CH2CI2. The filtrate was concentrated to afford the desired product. This material was recrystallized from hot hexanes to afford 14.5 g of Intermediate C4 as yellow needles. 1H-NMR (400 MHz, CDCI3): 8.02 (d, J = 9.2 Hz, 1 H), 7.45 (dd, J = 9.3, 2.8 Hz, 1 H), 7.32 (d, J = 2.7 Hz, 1 H), 6.59 - 6.43 (m, 1 H), 5.86 (dt, J = 17.3, 2.5 Hz, 1 H), 5.67 (d, J = 1 1 .0 Hz, 1 H), 3.98 (s, 3H) ppm.
References:
WO2014/145095,2014,A1 Location in patent:Page/Page column 111; 114-115
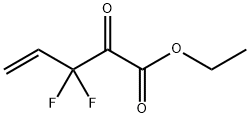
1294512-26-7
3 suppliers
inquiry

59548-39-9
116 suppliers
$10.00/1g

1535210-94-6
16 suppliers
inquiry
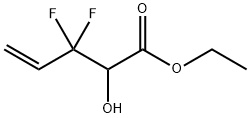
1365970-40-6
2 suppliers
inquiry

1535210-94-6
16 suppliers
inquiry

1294512-26-7
3 suppliers
inquiry

1535210-94-6
16 suppliers
inquiry

1535210-95-7
0 suppliers
inquiry

1535210-94-6
16 suppliers
inquiry