
Methyl 3-(1,3-dioxoisoindolin-2-yl)-2-hydroxypropanoate synthesis
- Product Name:Methyl 3-(1,3-dioxoisoindolin-2-yl)-2-hydroxypropanoate
- CAS Number:153744-36-6
- Molecular formula:C12H11NO5
- Molecular Weight:249.22

67-56-1
739 suppliers
$9.00/25ml
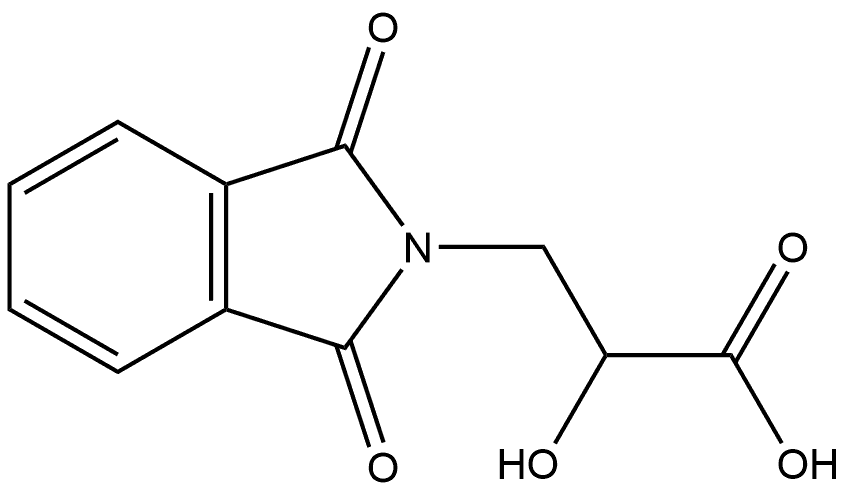
62983-58-8
0 suppliers
inquiry

153744-36-6
16 suppliers
$65.00/100mg
Yield:153744-36-6 1.56 g
Reaction Conditions:
in diethyl ether at 20;
Steps:
General procedure for the protection of the acyclic aminoacids II (GP5b)
General procedure: A solid, well-grounded mixture of the amino acid (1.0 eq)and phtalic anhydride (1.0 eq) was heated to 140 °C,resulting in a colourless melting. After 30 min, the reactionmixture was cooled to rt, solved in anhydrous MeOH(250 ml) and 2M HCl in Et2O (250 ml) was added. Themixture was stirred at rt until TLC indicated completeconsumption of the educt and reduced in vacuum. Thecrude compound was purified by flash column chromatographyon silica (eluent: CH2Cl2/EtOAc 9:1).
References:
Andre?, Janina C.;B?ck, Michael C.;H?fner, Georg;Wanner, Klaus T. [Medicinal Chemistry Research,2020,vol. 29,# 8,p. 1321 - 1340]
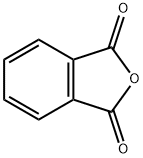
85-44-9
751 suppliers
$9.00/5g

153744-36-6
16 suppliers
$65.00/100mg