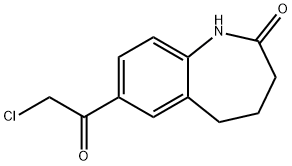
7-(chloroacetyl)-1,3,4,5-tetrahydro-2H-1-benzazepin-2-one synthesis
- Product Name:7-(chloroacetyl)-1,3,4,5-tetrahydro-2H-1-benzazepin-2-one
- CAS Number:154195-54-7
- Molecular formula:C12H12ClNO2
- Molecular Weight:237.68
Yield:154195-54-7 91%
Reaction Conditions:
Stage #1: chloroacetyl chloridewith carbon disulfide;aluminum (III) chloride for 0.25 h;Cooling with ice;
Stage #2: 2,3,4,5-tetrahydro-1H-1-benzo[b]azepin-2-one for 2.75 h;Reflux;
Steps:
Intermediate 12
To a 500 mL round-bottomed flask equipped with condenser and argon inlet were added aluminum chloride (5.79 g, 43.42 mmol), carbon disulfide (40 mL), and chloroacetyl chloride (1.68 g, 14.89 mmol) under ice-bath.
The mixture was stirred for 15 mins.
To the stirring mixture was added 4,5-dihydro-1H-benzo[b]azepine-2(3H)-one (2.00 g, 12.41 mmol) in portions over 5 mins.
The mixture was stirred for 10 minutes prior to reflux for 2.5 hours.
The reaction mixture was cooled and the solvent was decanted off.
Then ice and cold water (50 mL) was slowly added while stirred thoroughly.
The tan precipitate was collected by filtration and washed thoroughly with water.
The precipitate was sonicated in diethyl ether (10 mL) and the resulted fine solid was again collected by filtration.
The solid was dried under vacuum to give 7-(2-chloroacetyl)-1,3,4,5-tetrahydro-2H-benzo[b]azepin-2-one (2.68 g, 91%) as a beige solid. 1H NMR (400 MHz, DMSO-d): δ 9.90 (s, 1H), 7.90 (d, 1H, J=2.0 Hz), 7.85 (dd, 1H, J=8.0, 2.0 Hz), 7.07 (d, 1H, J=8.4 Hz), 2.76 (t, 2H, J=7.2 Hz), 2.20 (m, 2H), 2.16 (m, 2H). MS (ESI): Calcd. for C12H12ClNO2: 237, found 238 (M+1)+.
References:
US2018/86752,2018,A1 Location in patent:Paragraph 0078; 0079
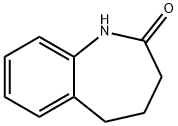
4424-80-0
143 suppliers
$25.00/1g

154195-54-7
5 suppliers
inquiry
