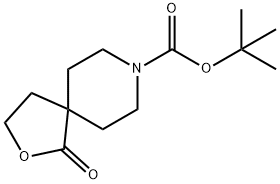
tert-butyl 1-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate synthesis
- Product Name:tert-butyl 1-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate
- CAS Number:154348-08-0
- Molecular formula:C13H21NO4
- Molecular Weight:255.31
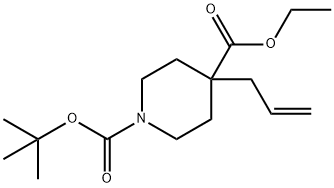
146603-99-8
60 suppliers
$45.00/100mg
![tert-butyl 1-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/154348-08-0.gif)
154348-08-0
36 suppliers
$117.00/50mg
Yield:154348-08-0 88%
Reaction Conditions:
Stage #1: 1-(1,1-dimethylethyl) 4-ethyl 4-(2-propenyl)-1,4-piperidinedicarboxylatewith oxygen;ozone in methanol;dichloromethane at -78; for 1.75 h;
Stage #2: with sodium tetrahydroborate in methanol;dichloromethane at -78 - 20; for 17 h;
Stage #3: in acetone at 20; for 0.166667 h;
Steps:
41 DESCRIPTION 41; 1,1-Dimethylethyl 1-Oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate
1-(1,1-Dimethylethyl) 4-ethyl 4-(2-propenyl)-1,4-piperidinedicarboxylate (Description 40, 20.0 g, 67.2 mmol) was dissolved in methanol (300 mL) and dichloromethane (300 mL) and cooled to -7820 C. Oxygen was bubbled through the solution for 10 minutes, then ozone for 75 minutes, to give a persistant blue coloration. Oxygen was bubbled through the solution for 10 minutes, then nitrogen for 10 minutes. Sodium borohydride (5.1 g, 135 mmol) was added and the mixture was stirred at -78° C. for 1 hour. Further sodium borohydride (5.1 g, 135 mmol) was added and the mixture was stirred at room temperature for 16 hours. Acetone (75 mL) was added and the mixture was stirred at room temperature for 10 minutes. Water (50 mL) was added and the organic solvent was evaporated under reduced pressure. Saturated aqueous ammonium chloride (500 mL) was added and the mixture was extracted with ethyl acetate (2×500 mL). The combined organic fractions were washed with aqueous citric acid (10%, 500 mL), saturated aqueous sodium hydrogen carbonate (500 mL) and brine (200 mL), dried (Na2SO4) and the solvent was evaporated under reduced pressure to give the title compound (15.0 g, 88%). 1H NMR (400 MHz, CDCl3) δ4.31 (2H, t, J 7 Hz), 3.97-3.87 (2H, m), 3.17-3.07 (2H, m), 2.20 (2H, t, J 7 Hz), 1.92-1.82 (2H, m), 1.60-1.45 (2H, m), and 1.45 (9H, s).
References:
US2003/225059,2003,A1 Location in patent:Page 27
374822-47-6
0 suppliers
inquiry
![tert-butyl 1-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/154348-08-0.gif)
154348-08-0
36 suppliers
$117.00/50mg
247133-32-0
5 suppliers
inquiry
![tert-butyl 1-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/154348-08-0.gif)
154348-08-0
36 suppliers
$117.00/50mg
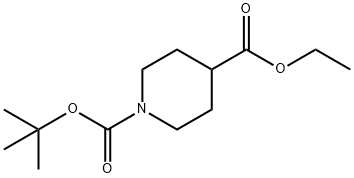
142851-03-4
296 suppliers
$5.00/1g
![tert-butyl 1-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/154348-08-0.gif)
154348-08-0
36 suppliers
$117.00/50mg
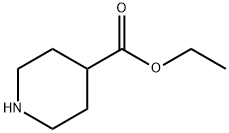
1126-09-6
481 suppliers
$15.40/25g
![tert-butyl 1-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate](/CAS/GIF/154348-08-0.gif)
154348-08-0
36 suppliers
$117.00/50mg