
2-Hydroxy-3,6,7,10,11-pentakis(hexyloxy)triphenylene synthesis
- Product Name:2-Hydroxy-3,6,7,10,11-pentakis(hexyloxy)triphenylene
- CAS Number:156244-98-3
- Molecular formula:C48H72O6
- Molecular Weight:745.08

94259-20-8
22 suppliers
$107.00/10g
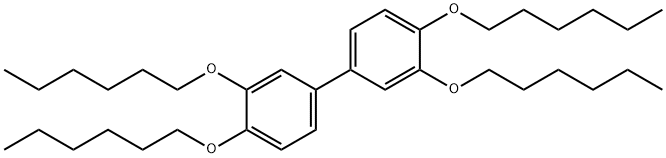
161691-13-0
0 suppliers
inquiry

156244-98-3
12 suppliers
$273.00/200MG
Yield: 82%
Reaction Conditions:
Stage #1:1,2-dihexyloxybenzene;3,3',4,4'-tetrahexyloxybiphenyl with iron(III) chloride in dichloromethane at 0 - 3; for 3 h;Scholl Reaction;
Stage #2: with water in methanol;dichloromethane
Steps:
Synthesis of MHT6 via a one-step cross-couplingbetween tetra- alkoxybiphenyl and dialkoxybenzene
To a vigorously stirred solution of 3,3’,4,4’-tetrahexyloxybiphenyl (0.555 g, 1 mmol) and 1,2-dihexyloxy- benzene (0.557 g, 2 mmol) in CH2Cl2(20 ml) at a temperature of 0-3°C (ice-water bath), anhydrous FeCl3 (4.866 g, 30 mmol) was added. The reaction mixture was kept stirring at 0-3°Cfor about 3 hours. CH3OH (20 ml) and water (200 ml) were then added successively to stop the reaction. The aqueous phase was extracted with CH2Cl2(15 ml) for 3-4 times. The organic layer was collected and dried with anhydrous MgSO4 or Na2SO4. Finally, the solvents were removed under reduced pressure and the crude product was purified by column chromatography. Elution of the column with 2-3% ethyl acetate in petroleum ether (60-90°C) afforded a pure product of MHT6 (0.611 g,82% yield). 1H NMR, 13CNMR and HRMS spectra were similar to MHT6synthesized above.
References:
Xiao, Weikang;He, Zhiqun;Xu, Min;Wu, Nan;Kong, Xiangfei;Jing, Xiping [Tetrahedron Letters,2015,vol. 56,# 5,p. 700 - 705] Location in patent:supporting information

94259-20-8
22 suppliers
$107.00/10g

156244-98-3
12 suppliers
$273.00/200MG
31189-03-4
2 suppliers
inquiry

94259-20-8
22 suppliers
$107.00/10g

156244-98-3
12 suppliers
$273.00/200MG

31189-03-4
2 suppliers
inquiry

94259-20-8
22 suppliers
$107.00/10g
![2,3,6,7,10,11-Hexakis[hexyloxy]triphenylene](/CAS/GIF/70351-86-9.gif)
70351-86-9
24 suppliers
$147.00/200mg

156244-98-3
12 suppliers
$273.00/200MG
![2,3,6,7,10,11-Hexakis[hexyloxy]triphenylene](/CAS/GIF/70351-86-9.gif)
70351-86-9
24 suppliers
$147.00/200mg

156244-98-3
12 suppliers
$273.00/200MG