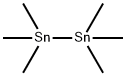2,7-Bis(trimethylstannyl)-benzo[1,2-b:4,5-b]bis(4,4'-dioctyl-4H-silolo[3,2-b]thiophene) synthesis
- Product Name:2,7-Bis(trimethylstannyl)-benzo[1,2-b:4,5-b]bis(4,4'-dioctyl-4H-silolo[3,2-b]thiophene)
- CAS Number:1569453-45-7
- Molecular formula:C44H74S2Si2Sn2
- Molecular Weight:960.79
1569453-37-7
0 suppliers
inquiry

1066-45-1
143 suppliers
$31.50/2g
![2,7-Bis(trimethylstannyl)-benzo[1,2-b:4,5-b]bis(4,4'-dioctyl-4H-silolo[3,2-b]thiophene)](/CAS/20211123/GIF/1569453-45-7.gif)
1569453-45-7
15 suppliers
inquiry
Yield:1569453-45-7 98.9%
Reaction Conditions:
Stage #1: benzo[1,2-b:4,5-b]bis(2-bromo-4,4’-dihexyl-4H-silolo[3,2-b]thiophene)with n-butyllithium in tetrahydrofuran at -78; for 0.166667 h;Inert atmosphere;
Stage #2: trimethyltin(IV)chloride in tetrahydrofuran at -78 - 20; for 4 h;Inert atmosphere;
Steps:
Synthesis of benzo[1,2-b:4,5-b]bis(2-trimethylstannyl-4,4’-dihexyl-4H-silolo[3,2-b]-thiophene) (3)
n-BuLi (1.6 M in hexane, 1.4 mL, 2.3 mmol) was injected dropwise via asyringe into a solution of benzo[1,2-b:4,5-b]-bis-(2-bromo-4,4’-dihexyl-4H-silolo[3,2-b]-thiophene (793 mg, 1.0 mmol) in anhydrous THF (100 mL) at -78 °C under N2. The mixturewas stirred at -78 °C for 10 min and then a solution of trimethyltin chloride (498.2 mg in 3.5mL dry THF, 2.5 mmol) was added quickly in one portion at -78 °C. The reaction was stirredat room temperature for another 4 hours. Water was added to the reaction mixture andextracted with hexanes. The combined organic layer was dried over anhydrous MgSO4 and thesolvent was removed in vacuo. The solid was precipitated out in iced MeOH solution andfiltered. Compound 3 was collected as pale yellow solid (950 mg, 98.9%).
References:
Love, John A.;Chou, Shu-Hua;Huang, Ye;Bazan, Guilllermo C.;Nguyen, Thuc-Quyen [Beilstein Journal of Organic Chemistry,2016,vol. 12,p. 2543 - 2555] Location in patent:supporting information
1217503-07-5
0 suppliers
inquiry
![2,7-Bis(trimethylstannyl)-benzo[1,2-b:4,5-b]bis(4,4'-dioctyl-4H-silolo[3,2-b]thiophene)](/CAS/20211123/GIF/1569453-45-7.gif)
1569453-45-7
15 suppliers
inquiry