
Ethyl 4-(((tert-butoxycarbonyl)amino)methyl)benzoate synthesis
- Product Name:Ethyl 4-(((tert-butoxycarbonyl)amino)methyl)benzoate
- CAS Number:157311-42-7
- Molecular formula:C15H21NO4
- Molecular Weight:279.33
Yield:157311-42-7 100%
Reaction Conditions:
with triethylamine in 1,4-dioxane;water at 0 - 20;
Steps:
Scheme S3. Synthesis of Ethyl4-(((tert-butoxycarbonyl)amino)methyl)benzoate (78)
Aminomethyl benzoic acid 79(560 mg, 2.98 mmol) was refluxed in 30mL of ethanol with 1 mL of HCl 12 N overnight. The solvent was evaporated toobtain aminomethyl benzoic ester 80as hydrochloride salt (640 mg, quantitative yield). The ester derivative (640mg, 2.97 mmol, 1 equiv) was dissolved in a dioxane/water solution, then TEA(911 μL, 6.53 mmol, 2.2 equiv) and Boc2O (779 mg, 3.57 mmol,1.2 equiv) were added and the solution was stirred at 0 C for 1 h, then overnight at rt. The solvent wasevaporated, the slurry was taken up in EtOAc, washed with a solution of citricacid 10% (3 X 10 mL), water (2 X 10 mL), dried over Na2SO4,filtered and evaporated in vacuum to give 78as oil (810 mg, quantitative yield). 1H NMR (200MHz, DMSO-d6) δ 7.88 (s, 2H), 7.40 (m, 1H), 7.32- 7.24 (m, 1H), 5.17(br, NH), 4.31-4.28 (m, 4 H), 1.39 (s, 9H), 1.34-1.30 (m, 3H) ppm.
References:
Roberti, Marinella;Schipani, Fabrizio;Bagnolini, Greta;Milano, Domenico;Giacomini, Elisa;Falchi, Federico;Balboni, Andrea;Manerba, Marcella;Farabegoli, Fulvia;De Franco, Francesca;Robertson, Janet;Minucci, Saverio;Pallavicini, Isabella;Di Stefano, Giuseppina;Girotto, Stefania;Pellicciari, Roberto;Cavalli, Andrea [European Journal of Medicinal Chemistry,2019,vol. 165,p. 80 - 92] Location in patent:supporting information

24424-99-5
841 suppliers
$13.50/25G
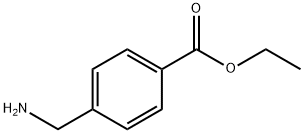
366-84-7
58 suppliers
$22.00/100mg

157311-42-7
14 suppliers
inquiry
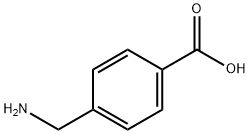
56-91-7
537 suppliers
$5.00/1g

157311-42-7
14 suppliers
inquiry
