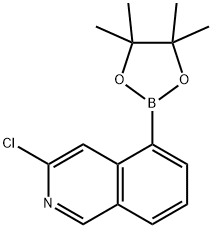
Isoquinoline, 3-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- synthesis
- Product Name:Isoquinoline, 3-chloro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
- CAS Number:1578245-90-5
- Molecular formula:C15 H17 B Cl N O2
- Molecular Weight:289.57
1029720-67-9
42 suppliers
$48.00/100mg

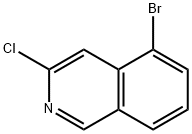
73183-34-3
564 suppliers
$6.00/5g

1578245-90-5
4 suppliers
inquiry
Yield:1578245-90-5 41%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in N,N-dimethyl-formamide at 100; for 1 h;Microwave irradiation;
Steps:
16 Preparation 16: 3-Chloro-5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)isoquinoline
Preparation 16: 3-Chloro-5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)isoquinoline A mixture of 5-bromo-3-c loroisoquinoline (63 mg, 0.41 mmol), KOAc (63 mg, 0.41 mmol), Pd(dppf)CI2 FontWeight="Bold" FontSize="10" DCM (22 mg, 0.03 mmol) and bis(pinacolato)diboron (63 mg, 0.41 mmol) in DMF (8 mL) was stirred at 100°C under microwave irradiation for 60 minutes. The reaction mixture was filtered, diluted with NaCI solution and extracted with EtOAc. The crude was purified by Biotage silica gel column chromatography eluting with 20% EtOAc/cyclohexane to afford the title compound as yellow oil (170 mg, 41 %). 1H NMR (500 MHz, CDCI3): δ 9.06 (d, J = 0.9 Hz, 1 H), 8.66 - 8.64 (m, 1 H), 8.31 (dd, J = 6.9, 1 .4 Hz, 1 H), 8.15 - 7.96 (m, 1 H), 7.60 (dd, J = 8.2, 6.9 Hz, 1 H), 1 .44 (s, 12H). LCMS (ESI) Rt = 3.02 minutes MS m/z 290 [M+H]+
References:
WO2014/37750,2014,A1 Location in patent:Paragraph 0012-0014