
4(3H)-Pyrimidinone, 2-amino-5-bromo-6-(trifluoromethyl)- synthesis
- Product Name:4(3H)-Pyrimidinone, 2-amino-5-bromo-6-(trifluoromethyl)-
- CAS Number:1583-00-2
- Molecular formula:C5H3BrF3N3O
- Molecular Weight:258
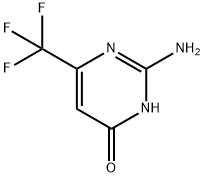
1513-69-5
142 suppliers
$14.00/1g

1583-00-2
0 suppliers
inquiry
Yield:1583-00-2 49%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 20; for 2 h;
Steps:
Step 1: 2-amino-5-bromo-4-(trifluoromethyl)-lH-pyrimidin-6-one
A solution of 2-amino-4-(trifluoromethyl)-lH-pyrimidin-6-one (2.5 g, 13.96 mmol) and '-b ro m o s ucci n i m i dc (2.61 g, 14.66 mmol) in MeCN (25 mL) was stirred at rt for 2h. The mixture was concentrated in vacuo, diluted with EtOAc and washed with water, satd. aq. NaHCCL and satd. aq. NaaSaCL solutions. The organic phase was dried over NaaSCL, fdtered and concentrated under vacuum to afford 2-amino-5-bromo-4-(trifluoromethyl)-lH- pyrimidin-6-one (1.75 g, 6.78 mmol, 49 % yield) as a yellow solid. LC/MS (ESI+) m/z = 258.0/260.0 [M+H]+.
References:
WO2021/108408,2021,A1 Location in patent:Page/Page column 87-88