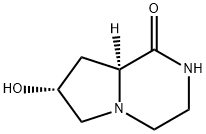
Pyrrolo[1,2-a]pyrazin-1(2H)-one, hexahydro-7-hydroxy-, (7R-cis)- (9CI) synthesis
- Product Name:Pyrrolo[1,2-a]pyrazin-1(2H)-one, hexahydro-7-hydroxy-, (7R-cis)- (9CI)
- CAS Number:158393-18-1
- Molecular formula:C7H12N2O2
- Molecular Weight:156.18
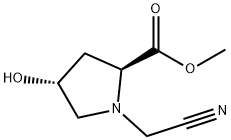
1429296-89-8
0 suppliers
inquiry
![Pyrrolo[1,2-a]pyrazin-1(2H)-one, hexahydro-7-hydroxy-, (7R-cis)- (9CI)](/CAS/GIF/158393-18-1.gif)
158393-18-1
7 suppliers
inquiry
Yield:158393-18-1 48%
Reaction Conditions:
with hydrogen in methanol at 80; under 1551.49 Torr; for 9 h;
Steps:
163.2
Step 2: (25',4R)-Methyl l-(cyanomethyl)-4-hydroxypyrrolidine-2- carboxylate (0.5 g, 2.71 mmol) from Step 1 and methanol (20 mL) were added to Raney- nickel 2800 water slurry (1.000 g, 17.04 mmol) in a 50 mL pressure bottle. The reaction mixture was stirred for 9 hours under hydrogen (30 psi) at 80 °C. The mixture was filtered though a nylon membrane, and the solvent was evaporated in vacuo. The resulting crude oil was purified on a 4 g silica gel cartridge eluted with 20% methanol/dichloromethane over 10 minutes to provide the crude solid product. Further purification on a 4 g cartridge with 20% methanol (2 N ammonia)/dichloromethane afforded (7R,8a5)-7-hydroxy- hexahydropyrrolo[l,2-a]pyrazin-l(2H)-one (0.204 g, 1.303 mmol, 48% yield) as a white solid. ? NMR (500 MHz, pyridine-i/5) ? ppm 8.44 (bs, 1H), 6.57 (d, J = 4.0 Hz, 1H), 4.68 (d, J = 3.2 Hz, 1H), 3.86 (t, J = 8.2 Hz, 1H), 3.54 - 3.45 (m, 1H), 3.30 (dd, J = 10.0, 5.8 Hz, 1H), 3.28 - 3.23 (m, 1H), 2.95 (dd, J = 9.9, 3.6 Hz, 1H), 2.88 (dt, J = 12.0, 4.2 Hz, 1H), 2.79 (ddd, J = 12.1, 9.4, 4.2 Hz, 1H), 2.61 - 2.55 (m, 2H); MS (ESI+) m/z 157 (M+H)+.
References:
WO2013/49164,2013,A1 Location in patent:Paragraph 1091