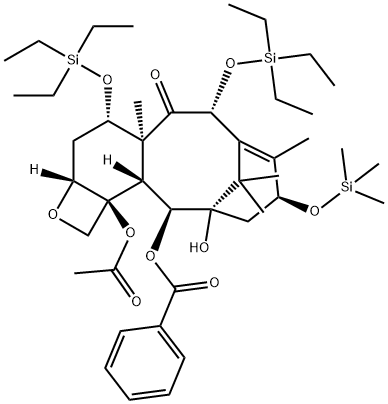
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(Acetyloxy)-12-(benzoyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-4,6-bis[(triethylsilyl)oxy]-9-[(trimethylsilyl)oxy]-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one synthesis
- Product Name:(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(Acetyloxy)-12-(benzoyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-4,6-bis[(triethylsilyl)oxy]-9-[(trimethylsilyl)oxy]-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one
- CAS Number:159383-93-4
- Molecular formula:C44H72O10Si3
- Molecular Weight:845.29
75-77-4
564 suppliers
$5.04/10
![7,10-Bis[O-(triethylsilyl)]-10-deacetyl Baccatin III](/CAS/GIF/149107-84-6.gif)
149107-84-6
11 suppliers
$139.90/1mg
![(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(Acetyloxy)-12-(benzoyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-4,6-bis[(triethylsilyl)oxy]-9-[(trimethylsilyl)oxy]-7,11-methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one](/CAS/GIF/159383-93-4.gif)
159383-93-4
11 suppliers
$218.09/1mg
Yield:159383-93-4 96.5%
Reaction Conditions:
with 1H-imidazole;dmap in dichloromethane at 0; for 0.5 h;
Steps:
14
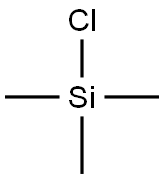
13-O-Trimethylsilyl-7,10-bis-O-triethylsilyl-10-deacetyl baccatin (III).. To a solution of 0.5 g (0.66 mmol) of 7,10-bis-O-triethylsilyl-10-deacetyl baccatin (III), 90 mg (2 eq) of imidazole, 40 mg (0.5 eq) of p-dimethylaminopyridine (DMAP) in 15 ML of CH2Cl2 at 0° C. was added 0.17 ML (2 eq) of trimethylsilyl chloride (TMSCl).. The solution was stirred at 0° C. for 30 min and 1.0 ML of methanol was added.. The mixture was diluted with H2O (10.0 ML and EtOAc (50.0 ML) and the organic layer was separated, washed with brine (10.0 ML), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to afford a crude solid (0.58 g).. Plug filtration with 10% EtOAc in hexane gave 13-O-trimethylsilyl-7,10-bis-O-triethylsilyl-10-deacetyl baccatin (III) (0.53 g, 96.5%).. m.p. 213-215° C., [α]25Na -43.0° (c 0.5, CHCl3), 1H NMR (CDCl3, 300 MHz), δ 8.10 (d, J=7.1 Hz, 2H, benzoate ortho), 7.6-7.4 (m, 3H, aromatic), 5.62 (d, J=7.1 Hz, 1H, H2), 5.19 (s, 1H, H10), 4.94 (dd, J=1.8, 8.8 Hz, 1H, H5), 4.86 (m, 1H, H13), 4.41 (dd, J=6.6, 10.4 Hz, 1H, H7), 4.28 (d, J=8.2 Hz, 1H, H20α), 4.12 (d, J=8.2 Hz, 1H, H20β), 3.86 (d, J=7.14 Hz, 1H, H3), 2.51 (m, 1H, H6α), 2.26 (s, 3H, 4Ac), 2.22-2.03 (m, 2, H14α, H14β), 1.93 (s, 3H, Me18), 1.84 (m, 1H, H6β), 1.64 (s, 3H, Me19), 1.19 (s, 3H, Me17), 1.12 (s, 3H, Me16), 1.02-0.93 (m, 18H, SiCH2CH3), 0.69-0.56 (m, 12H, SiCH2CH3), 0.17 (s, 9H, SiCH3).
References:
US6727369,2004,B1 Location in patent:Page column 39 - 40