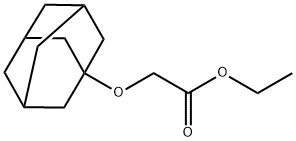
Acetic acid, 2-(tricyclo[3.3.1.13,7]dec-1-yloxy)-, ethyl ester synthesis
- Product Name:Acetic acid, 2-(tricyclo[3.3.1.13,7]dec-1-yloxy)-, ethyl ester
- CAS Number:159847-42-4
- Molecular formula:C14H22O3
- Molecular Weight:238.32
![Acetic acid, 2-(tricyclo[3.3.1.13,7]dec-1-yloxy)-, ethyl ester](/CAS/20210305/GIF/159847-42-4.gif)
159847-42-4
0 suppliers
inquiry

1614-38-6
13 suppliers
$85.00/250mg
Yield:1614-38-6 92%
Reaction Conditions:
with potassium hydroxide in n-propanol water;ethyl acetate;
Steps:
1.a 2-[5-(Adamantan-1-yloxymethyl)-2-cyclohexyl-1H-imidazole-4-yl]-benzooxazole-5-carboxylic acid
Step a. (Adamantan-1-yloxy)-acetic acid. A mixture of (adamantan-1-yloxy)-acetic acid ethyl ester (A.F. Noels et al. Tetrahedron, 1982, 38, 2733) (7.29 g, 29 mmol) and potassium hydroxide (2.60 g, 46 mmol) in water-ethanol (1:2 mixture, 180 ml) was heated at reflux for 2 h. The mixture was cooled, then concentrated in vacuum and acidified with concentrated hydrochloric acid. The resultant white precipitate was dissolved in ethyl acetate (200 ml). The solution was washed with brine (2*200 ml), dried (MgSO4) and the solvent was evaporated to afford a white crystalline solid (5.85 g 92%). 1H NMR (300 MHz, CDCl3) 4.08 (2H, s), 2.20-1.58 (15H, m).
References:
US2003/191116,2003,A1
![Acetic acid, 2-(tricyclo[3.3.1.13,7]dec-1-yloxy)-, ethyl ester](/CAS/20210305/GIF/159847-42-4.gif)
159847-42-4
0 suppliers
inquiry
![Butanoic acid, 3-oxo-4-(tricyclo[3.3.1.13,7]dec-1-yloxy)-2-(triphenylphosphoranylidene)-, phenylmethyl ester](/CAS/20210305/GIF/269071-39-8.gif)
269071-39-8
0 suppliers
inquiry