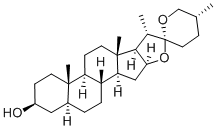
16-Allopregnen-3beta-ol-20-one synthesis
- Product Name:16-Allopregnen-3beta-ol-20-one
- CAS Number:566-61-0
- Molecular formula:C21H32O2
- Molecular Weight:316.48
Yield:566-61-0 92% ,2857-75-2 80%
Reaction Conditions:
Stage #1: tigogeninwith acetic anhydride in acetic acid at 200; for 1 h;
Stage #2: with dihydrogen peroxide;sodium phosphotungstate in water;butan-1-ol at 80; for 2 h;
Stage #3: with sodium hydroxide in water;butan-1-ol; for 2 h;Product distribution / selectivity;Heating / reflux;
Steps:
9
Example 9 Degradation of tigogenin to 3β-hydroxy-5α-pregn-16(17)-ene-20-one and 4R-methyl-δ-pentyl lactone 100 g of tigogenin, dissolved in acetic acid and acetic anhydride, was kept in the pressure kettle at 200° C. for one hour, then the low boilers were removed under reduced pressure and the residue was dissolved in 500 ml of BuOH. 200 mg of Na3[P(W12O40)] and 50 ml of hydrogen peroxide (30% H2O) were added, and this mixture was stirred for 2 hours at 80° C. After NaOH was added, it was kept on refluxing for 2 hours. The mixture was concentrated, diluted with water, and filtrated to get 70 g of 3β-hydroxy-5α-pregn-16(17)-ene-20-one in 92% yield. m.p. 207-9° C., [α]20D=+51° (c 0.9 CHCl3), 1H-NMR (300 MHz, CDCl3)δ(ppm): 6.59 (dd, J=1.3 Hz, 1H, 16-H), 3.45 (m, 1H, 3-H), 2.26 (s, 3H, CH3CO-, 21-H), 0.83 (s, 3H, 18-H), 0.89 (s, 3H, 19-H). MS (m/z, %): 316 (M+), 301 (M+-CH3), 283 (M+-CH3-H2O), 159, 145, 115, 105, 91, 43. The water layer was acidified and extracted with organic solvent to give 21 g of 4R-methyl-δ-pentyl lactone in 80% yield.
References:
US2006/166955,2006,A1 Location in patent:Page/Page column 4
![Acetamide, N-[(3β,5α,25S)-3-(acetyloxy)furost-20(22)-en-26-yl]-](/CAS/20210305/GIF/2857-74-1.gif)
2857-74-1
0 suppliers
inquiry

566-61-0
11 suppliers
inquiry
1169-20-6
31 suppliers
inquiry

566-61-0
11 suppliers
inquiry
2309-38-8
0 suppliers
inquiry

566-61-0
11 suppliers
inquiry
77-59-8
108 suppliers
$60.00/25mg

566-61-0
11 suppliers
inquiry