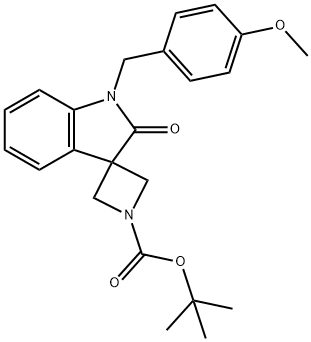
tert-Butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indoline]-1-carboxylate synthesis
- Product Name:tert-Butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indoline]-1-carboxylate
- CAS Number:1603067-25-9
- Molecular formula:C23H26N2O4
- Molecular Weight:394.46
![1-Azetidinecarboxylic acid, 3-[[(2-bromophenyl)[(4-methoxyphenyl)methyl]amino]carbonyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1603067-24-8.gif)
1603067-24-8
0 suppliers
inquiry
![tert-Butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indoline]-1-carboxylate](/CAS/20210111/GIF/1603067-25-9.gif)
1603067-25-9
3 suppliers
inquiry
Yield:1603067-25-9 68.6%
Reaction Conditions:
with [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II) dichloride;sodium t-butanolate in toluene at 110; for 0.5 h;Microwave irradiation;
Steps:
1.3 tert-butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indol]-1-carboxylate
The tert-butyl 3-((2-bromophenyl)(4-methoxybenzyl)carbamoyl)azetidine-1-carboxylate(1.63 g, 3.42 _1 ) was dissolved in 5 mL of dry toluene, was added palladium catalyst i-Pr-PEPPSI9 (163 mg, CAS: 905459-27-0), sodium tert-butoxide (493 mg, 5.13 mmol), stirred at 110 ° C microwave for 30 min. The reaction solution was added 100 mL of water, extracted with ethyl acetate (100 mLx2), Merger organic phase was washed with water 100 ml x 3), saturated sodium chloride solution (100 mL x 2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure, by silica column chromatography eluting with system B agent resulting residue was purified to give tert-butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indol]-1-carboxylate(3.7g, colorless oil) yield: 68.6%.
References:
TW2016/5834,2016,A Location in patent:Paragraph 0160; 0165; 0166
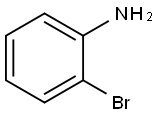
615-36-1
406 suppliers
$10.00/5g
![tert-Butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indoline]-1-carboxylate](/CAS/20210111/GIF/1603067-25-9.gif)
1603067-25-9
3 suppliers
inquiry
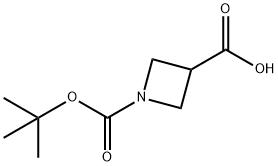
142253-55-2
317 suppliers
$6.00/1g
![tert-Butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indoline]-1-carboxylate](/CAS/20210111/GIF/1603067-25-9.gif)
1603067-25-9
3 suppliers
inquiry

1603067-22-6
1 suppliers
inquiry
![tert-Butyl 1'-(4-methoxybenzyl)-2'-oxospiro[azetidine-3,3'-indoline]-1-carboxylate](/CAS/20210111/GIF/1603067-25-9.gif)
1603067-25-9
3 suppliers
inquiry