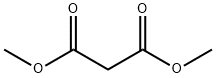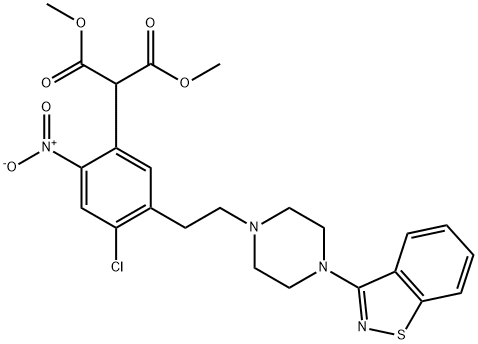
2-[5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-4-chloro-2-nitrophenyl]-propanedioic Acid 1,3-DiMethyl Ester synthesis
- Product Name:2-[5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-4-chloro-2-nitrophenyl]-propanedioic Acid 1,3-DiMethyl Ester
- CAS Number:160384-39-4
- Molecular formula:C24H25ClN4O6S
- Molecular Weight:533
Yield:160384-39-4 45%
Reaction Conditions:
with potassium hydroxide;nitrogen in 1-Methylpyrrolidine;
Steps:
5 5-Chloro-4-(2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl)-2-(bis-(methoxycarbonyl)methyl)-nitrobenzene
EXAMPLE 5 5-Chloro-4-(2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl)-2-(bis-(methoxycarbonyl)methyl)-nitrobenzene 2,5-Dichloro-4-(2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl)-nitrobenzene (2.4 g, 55 mmol) and dimethylmalonate (1.81 g, 137 mmol) were dissolved in N-methyl-pyrrolidine (24 ml) and a stream of nitrogen was bubbled through the solution for 15 minutes to remove any oxygen. Powdered potassium hydroxide (0.77 g, 137 mmol) was added in one portion and the mixture heated at 40°-5° C. for 5 hours. The reaction mixture was cooled and diluted with ethyl acetate and washed with saturated ammonium chloride solution, water, and brine. The organic layer was dried over magnesium sulfate, filtered and evaporated to afford the crude product. The pure product was isolated by flash chromatography over silica gel with ethyl acetate/hexanes (4:6) as a light yellow solid, 1.32 g, 45% yield. mp 128°-31° C. NMR (CDCl3) δ 8.10 (s,1), 7.90 (d, 1), 7.81 (d, 1), 7.48 (t and s, 2), 7.37 (t, 1), 5.33 (s,1), 3.82 (s,6), 3.58 (m, 4), 3.06 (t, 2), 2.80-2.70 (m, 6). The structure was confirmed by single crystal x-ray analysis.
References:
US5359068,1994,A
19398-61-9
138 suppliers
$10.00/5G
![2-[5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-4-chloro-2-nitrophenyl]-propanedioic Acid 1,3-DiMethyl Ester](/CAS/GIF/160384-39-4.gif)
160384-39-4
12 suppliers
inquiry

7149-76-0
14 suppliers
$28.60/50MG
![2-[5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-4-chloro-2-nitrophenyl]-propanedioic Acid 1,3-DiMethyl Ester](/CAS/GIF/160384-39-4.gif)
160384-39-4
12 suppliers
inquiry
![3-[4-[2-(2,5-Dichloro-4-nitrophenyl)ethyl]-1-piperazinyl]-1,2-benzisothiazole](/CAS/GIF/160384-38-3.gif)