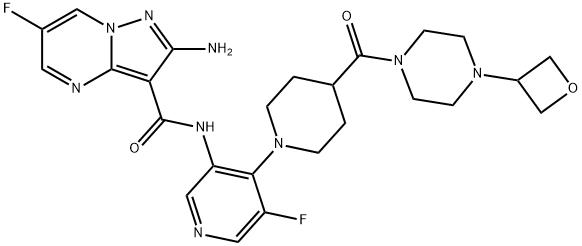
2-amino-6-fluoro-N-(5-fluoro-4-(4-(4-(oxetan-3-yl)piperazine-1-carbonyl)piperidin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide(WXC04788) synthesis
- Product Name:2-amino-6-fluoro-N-(5-fluoro-4-(4-(4-(oxetan-3-yl)piperazine-1-carbonyl)piperidin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide(WXC04788)
- CAS Number:1613191-99-3
- Molecular formula:C25H29F2N9O3
- Molecular Weight:541.55
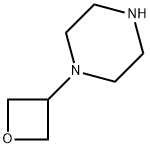
1254115-23-5
118 suppliers
$33.00/250mg
![4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-, hydrochloride (1:1)](/CAS/20211123/GIF/1613192-04-3.gif)
1613192-04-3
0 suppliers
inquiry
![2-amino-6-fluoro-N-(5-fluoro-4-(4-(4-(oxetan-3-yl)piperazine-1-carbonyl)piperidin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide(WXC04788)](/CAS/20200515/GIF/1613191-99-3.gif)
1613191-99-3
27 suppliers
inquiry
Yield: 86%
Reaction Conditions:
with N-[(6-chlorobenzotriazol-1-yl)oxy-(dimethylamino)methylene]-dimethyl-ammonium tetrafluoroborate;N-ethyl-N,N-diisopropylamine in tetrahydrofuranInert atmosphere;Solvent;
Steps:
3f.4; 3f.3 Step 4: 2-amino-6-fluoro-N-[5-fluoro-4-[4-[4-(oxetan-3-yl)piperazine-1-carbonyl]-1- piperidyl]-3-pyridyI]pyrazolo[1,5-a]pyrimidine-3-carboxamide (Compound I-G-32)
To a yellow suspension of l-[3-[(2-amino-6-fluoro-pyrazolo[l,5-a]pyrimidine-3- carbonyl)amino]-5-fluoro-4-pyridyl]piperidine-4-carboxylic acid (Hydrochloric Acid) 30 (59.7 g, 131.5 mmol) in NMP (477.6 mL) was added DIPEA (50.99 g, 68.72 mL, 394.5 mmol) then [(6-chlorobenzotriazol- 1 -yl)oxy-(dimethylamino)methylene] -dimethyl- ammonium (Boron Tetrafluoride Ion (1)) (51.44 g, 144.7 mmol). A yellow suspension forms after a few minutes. The mixture was sirred for 30 mins at room temperature then l-(oxetan- 3-yl)piperazine 25 (prepared according to Preparation N-32, below) (26.18 g, 184.1 mmol) was added. The cream/tan suspension turns to an orange solution (exotherms from 23.9 to 29.4°C). The flask was placed on ice/water bath until internal temperature was at 24°C, then ice bath was removed and internal temperature steady at 24°C thereafter. [00330] The solution was stirred for 30 mins at room temperature then cooled on an ice/salt/water bath to 10°C before the slow addition of water (1.015 L) in 100 mL portions. Prior to adding the next lOOmL of water, waited for exotherm to between 17°C and 20°C (internal) then allow to cool to between 10 and 15°C. Repeated until all water added. Once exotherm had ceased, ice/salt/water bath removed and mixture stirred at ambient temperature for 20 mins (thick yellow/cream suspension forms). Solid collected by filtration through a sinter funnel, washed well with water then dried by suction for 10 mins. Vacuum removed and solid slurried in water on sinter funnel, then vacuum reapplied and solid dried by suction overnight then dried in vacuum oven for 24 h at 40°C <10 mBar. [00331] Solid (54.5g) suspended in ethanol (545 mL, 10 vol eq.) and heated under reflux for 2h then cooled to room temperature over 2h. Solid collected by filtration, washed with minimum ethanol and dried by suction for lh to leave product as a pale yellow solid. Solid placed in vacuum oven at 23.5°C and <10mBar overnight to leave product I-G-32 as a pale yellow solid, (51g, 64% yield). 'H NMR (500 MHz, DMSO-d6) δ 10.64 (s, 1H), 9.67 (s, 1H), 9.48 (dd, 1H), 9.26 (dd, 1H), 8.26 (d, 1H), 6.79 (s, 2H), 4.55 (t, 2H), 4.47 (t, 2H), 4.34 (t, 0.7H), 3.61 (dt, 4H), 3.48 - 3.41 (m, 2.5H), 3.22 - 3.17 (m, 2H), 3.05 - 3.03 (m, 2H), 3.99 - 2.93 (m, 1H), 2.28 (dt, 4H), 2.17 - 2.10 (m, 2H), 1.74 (d, 2H), 1.07 (t, 2H). MS (ES+) 542.3.; To a solution of l-(oxetan-3-yl)piperazine (525mg, 3.69mmol) in THF (12ml) was added DIPEA (1.72ml, 9.91mmol), followed by l-(3-(2-amino-6-fluoropyrazolo[l,5- a]pyrimidine-3 -carboxamido)-5 -fluoropyridin-4-yl)piperidine-4-carboxylic acid(hydrochloride salt, 1.5g, 3.3mmol). [(6-chlorobenzotriazol-l-yl)oxy- (dimethylamino)methylene]-dimethyl-ammonium tetrafluoroborate (TCTU, 1.29g,3.64mmol) was added and the mixture stirred under nitrogen until reaction completion (determined by HPLC analysis). The mixture was cooled and water (24ml) was added dropwise. The mixture was allowed to warm to ambient and stirred for 3 hrs, then filtered. The filter cake was washed with (3x3 ml). The damp cake was dried under vacuum (with a nitrogen bleed) at 40°C. The product was obtained as a yellow solid (1.54g, 86%); XH NMR (500 MHz, DMSO-d6) δ 10.64 (s, 1H), 9.67 (s, 1H), 9.48 (dd, 1H), 9.26 (dd, 1H), 8.26 (d, 1H), 6.79 (s, 2H), 4.55 (t, 2H), 4.47 (t, 2H), 4.34 (t, 0.7H), 3.61 (dt, 4H), 3.48 - 3.41 (m, 2.5H), 3.22 - 3.17 (m, 2H), 3.05 - 3.03 (m, 2H), 3.99 - 2.93 (m, 1H), 2.28 (dt, 4H), 2.17 - 2.10 (m, 2H), 1.74 (d, 2H), 1.07 (t, 2H); 19F NMR (500 MHz, DMSO-d6) δ - 152.8, -136.1 ; MS (ES+) 542.3.
References:
VERTEX PHARMACEUTICALS INCORPORATED;AHMAD, Nadia;BOYALL, Dean;CHARRIER, Jean-Damien;DAVIS, Chris;DAVIS, Rebecca;DURRANT, Steven;ETXEBARRIA I JARDI, Gorka;FRAYSSE, Damien;JIMENEZ, Juan-Miguel;KAY, David;KNEGTEL, Ronald;MIDDLETON, Donald;ODONNELL, Michael;PANESAR, Maninder;PIERARD, Francoise;PINDER, Joanne;SHAW, David;STORCK, Pierre-Henri;STUDLEY, John;TWIN, Heather WO2014/89379, 2014, A1 Location in patent:Paragraph 00329 - 00331; 00334
407-20-5
349 suppliers
$10.00/1g
![2-amino-6-fluoro-N-(5-fluoro-4-(4-(4-(oxetan-3-yl)piperazine-1-carbonyl)piperidin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide(WXC04788)](/CAS/20200515/GIF/1613191-99-3.gif)
1613191-99-3
27 suppliers
inquiry
1211540-92-9
70 suppliers
$45.00/50mg
![2-amino-6-fluoro-N-(5-fluoro-4-(4-(4-(oxetan-3-yl)piperazine-1-carbonyl)piperidin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide(WXC04788)](/CAS/20200515/GIF/1613191-99-3.gif)
1613191-99-3
27 suppliers
inquiry

1613192-96-3
13 suppliers
inquiry
![2-amino-6-fluoro-N-(5-fluoro-4-(4-(4-(oxetan-3-yl)piperazine-1-carbonyl)piperidin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide(WXC04788)](/CAS/20200515/GIF/1613191-99-3.gif)
1613191-99-3
27 suppliers
inquiry
![Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid, 2-amino-6-fluoro-, 2-propen-1-yl ester](/CAS/20210111/GIF/1613191-76-6.gif)
1613191-76-6
1 suppliers
inquiry
![2-amino-6-fluoro-N-(5-fluoro-4-(4-(4-(oxetan-3-yl)piperazine-1-carbonyl)piperidin-1-yl)pyridin-3-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide(WXC04788)](/CAS/20200515/GIF/1613191-99-3.gif)
1613191-99-3
27 suppliers
inquiry