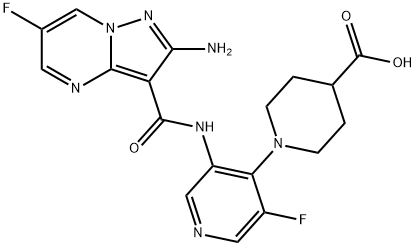
4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]- synthesis
- Product Name:4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-
- CAS Number:1613192-02-1
- Molecular formula:C18H17F2N7O3
- Molecular Weight:417.37
![4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1613192-00-9.gif)
1613192-00-9
0 suppliers
inquiry
![4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-](/CAS/20200611/GIF/1613192-02-1.gif)
1613192-02-1
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 20; for 5 h;
Steps:
4.1 Step 1: 1-(3-(2-amino-6-fluoropyrazolo[1,5-a]pyrimidine-3-carboxamido)-5-fluoropyridin-4-yl)piperidine-4-carboxylic acid (Compound Ι-Ν-92)
tert-butyl l-(3-(2-amino-6-fluoropyrazolo[l,5-a]pyrimidine-3-carboxamido)pyridin- 4-yl)piperidine-4-carboxylate prepared according to methods similar to the one depicted in Example 1 was dissolved in DCM (5mL). TFA (1 mL, 12.98 mmol) was added and the mixture was stirred at RT for 5 hr. The reaction mixture was concentrated to give l-(3-(2- amino-6-fluoropyrazolo[l,5-a]pyrimidine-3-carboxamido)-5-fluoropyridin-4-yl)piperidine-4- carboxylic acid as a beige solid that was used in next step without further purification. MS (ES+) 418.1.
References:
WO2014/89379,2014,A1 Location in patent:Paragraph 00446
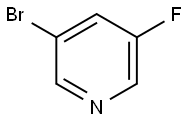
407-20-5
339 suppliers
$10.00/1g
![4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-](/CAS/20200611/GIF/1613192-02-1.gif)
1613192-02-1
0 suppliers
inquiry
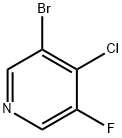
1211540-92-9
67 suppliers
$45.00/50mg
![4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-](/CAS/20200611/GIF/1613192-02-1.gif)
1613192-02-1
0 suppliers
inquiry

1613192-96-3
12 suppliers
inquiry
![4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-](/CAS/20200611/GIF/1613192-02-1.gif)
1613192-02-1
0 suppliers
inquiry
![Pyrazolo[1,5-a]pyrimidine-3-carboxylic acid, 2-amino-6-fluoro-, 2-propen-1-yl ester](/CAS/20210111/GIF/1613191-76-6.gif)
1613191-76-6
0 suppliers
inquiry
![4-Piperidinecarboxylic acid, 1-[3-[[(2-amino-6-fluoropyrazolo[1,5-a]pyrimidin-3-yl)carbonyl]amino]-5-fluoro-4-pyridinyl]-](/CAS/20200611/GIF/1613192-02-1.gif)
1613192-02-1
0 suppliers
inquiry