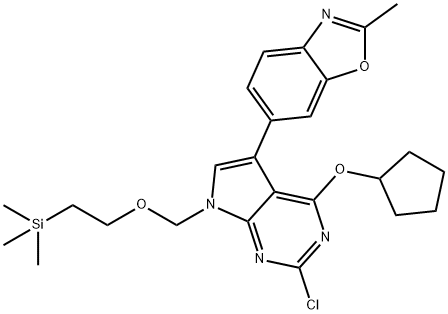
7H-Pyrrolo[2,3-d]pyrimidine, 2-chloro-4-(cyclopentyloxy)-5-(2-methyl-6-benzoxazolyl)-7-[[2-(trimethylsilyl)ethoxy]methyl]- synthesis
- Product Name:7H-Pyrrolo[2,3-d]pyrimidine, 2-chloro-4-(cyclopentyloxy)-5-(2-methyl-6-benzoxazolyl)-7-[[2-(trimethylsilyl)ethoxy]methyl]-
- CAS Number:1618663-22-1
- Molecular formula:C25H31ClN4O3Si
- Molecular Weight:499.08
Yield:1618663-22-1 88%
Reaction Conditions:
with sodium t-butanolate in 1,3-dioxane at 70; for 3 h;Inert atmosphere;Sealed tube;
Steps:
3 6-(2-Chloro-4-(cyclopentyloxy)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-2-methylbenzo[d]oxazole
A solution of 6-(2,4-dichloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-2-methylbenzo[d]oxazole (1 equiv), cyclopentanol (1.1 equiv), and sodium tert-butoxide (1 equiv) in 1,4-dioxane (0.22 M) was taken in a sealable flask. The reaction mixture was put under a nitrogen atmosphere, the sealable flask was sealed and the mixture was stirred at 70° C. for 3 h. The mixture was cooled to room temperature and concentrated to afford an oil, which was suspended in DCM and loaded onto a silica column (packed with hexane). The column was eluted with 0-30% ethyl acetate in hexane. The fractions containing product were combined and concentrated to dryness to give the target product as a light yellow oil (Yield: 88%) that solidified upon standing. MS (ESI) m/z 499.4 [M+1]+
References:
US2014/200206,2014,A1 Location in patent:Paragraph 0232
![2-Methylbenzo[d]oxazol-6-ylboronic acid pinacol ester](/CAS/20150408/GIF/1408089-23-5.gif)
1408089-23-5
46 suppliers
inquiry
![2-Chloro-4-(cyclopentyloxy)-5-iodo-7-[[2-(trimethylsilyl)ethoxy]methyl]-7H-pyrrolo[2,3-d]pyrimidine](/CAS/20210111/GIF/1618663-33-4.gif)
1618663-33-4
12 suppliers
inquiry
![7H-Pyrrolo[2,3-d]pyrimidine, 2-chloro-4-(cyclopentyloxy)-5-(2-methyl-6-benzoxazolyl)-7-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210111/GIF/1618663-22-1.gif)
1618663-22-1
0 suppliers
inquiry
![2,4-Dichloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine](/CAS2/GIF/1012785-51-1.gif)
1012785-51-1
83 suppliers
$11.00/1g
![7H-Pyrrolo[2,3-d]pyrimidine, 2-chloro-4-(cyclopentyloxy)-5-(2-methyl-6-benzoxazolyl)-7-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210111/GIF/1618663-22-1.gif)
1618663-22-1
0 suppliers
inquiry
![7-((2-(triMethylsilyl)ethoxy)Methyl)-2,4-dichloro-5-iodo-7H-pyrrolo[2,3-d]pyriMidine](/CAS/20150408/GIF/1404364-72-2.gif)
1404364-72-2
31 suppliers
inquiry
![7H-Pyrrolo[2,3-d]pyrimidine, 2-chloro-4-(cyclopentyloxy)-5-(2-methyl-6-benzoxazolyl)-7-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210111/GIF/1618663-22-1.gif)
1618663-22-1
0 suppliers
inquiry
151230-42-1
96 suppliers
$13.00/250mg
![7H-Pyrrolo[2,3-d]pyrimidine, 2-chloro-4-(cyclopentyloxy)-5-(2-methyl-6-benzoxazolyl)-7-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210111/GIF/1618663-22-1.gif)
1618663-22-1
0 suppliers
inquiry
![7H-Pyrrolo[2,3-d]pyrimidine, 2,4-dichloro-5-(2-methyl-6-benzoxazolyl)-7-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210111/GIF/1618663-21-0.gif)