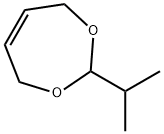
1,5-dihydro-3-isopropyl-8-methyl-[1,3]dioxepino[5,6-c]pyridin-9-ol synthesis
- Product Name:1,5-dihydro-3-isopropyl-8-methyl-[1,3]dioxepino[5,6-c]pyridin-9-ol
- CAS Number:1622-67-9
- Molecular formula:C12H17NO3
- Molecular Weight:223.27
![6,9-Epoxy[1,3]dioxepino[5,6-c]pyridine, 9-ethoxy-1,5,5a,6,9,9a-hexahydro-8-methyl-3-(1-methylethyl)-](/CAS/20210305/GIF/5205-63-0.gif)
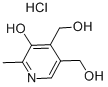
5205-63-0
0 suppliers
inquiry
![1,5-dihydro-3-isopropyl-8-methyl-[1,3]dioxepino[5,6-c]pyridin-9-ol](/CAS/GIF/1622-67-9.gif)
1622-67-9
7 suppliers
inquiry
Yield:1622-67-9 98%
Reaction Conditions:
with glacial acetic acid at 80 - 92; under 225.023 - 760.051 Torr; for 2.75 h;
Steps:
5
The crude Diels-Alder product mixture weighing 92.4 g (279.3 mmol, containing 225.7 mmol of ADDI, 65.8 w/w% GC, and 53.6 mmol of PIB, 13.0 w/w% GC) was heated in a water bath at80 C until the mixture had become fluid, the heating time being approximately 30 minutes. To the stirred reaction mixture (100 rpm) at80 C were added dropwise 15 ml of acetic acid (263 mmol, 1.16 equivalents calculated from ADDI) through Metrohm Dosimat over 15 minutes. After the addition of acetic acid, a reduced pressure of 300 mbar (30 kPa) was applied to the reactor and the low boiling products, mainly ethanol, were trapped in a Liebig condenser at-78 C during the course of the reaction. After the addition of the acid, an exothermic reaction was observed and the internal temperature of the reaction mixture reached about92 C. The mixture was stirred for 2 hours at80 C and monitored by GC (10 mg samples of reaction mixture dissolved in 1 ml of ethyl acetate containing 1% of triethylamine). After 2 hours, no ADDI starting material was observed by GC (less than 0.1 w/w%). PIB was observed by GC as the only major product (60.7 w/w% GC, 273.7 mmol). From the GC analysis, the yield of the rearrangement was 98%. The crude PIB ("rearrangement mixture") was used directly in the next process step described in Example 6 below.
References:
WO2005/49618,2005,A1 Location in patent:Page/Page column 14
![6,9-Epoxy[1,3]dioxepino[5,6-c]pyridine, 9-butoxy-1,5,5a,6,9,9a-hexahydro-8-methyl-3-(1-methylethyl)-](/CAS/20210305/GIF/717916-87-5.gif)
717916-87-5
0 suppliers
inquiry
![1,5-dihydro-3-isopropyl-8-methyl-[1,3]dioxepino[5,6-c]pyridin-9-ol](/CAS/GIF/1622-67-9.gif)
1622-67-9
7 suppliers
inquiry
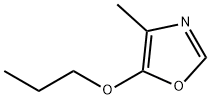
5006-20-2
124 suppliers
$21.00/250mg
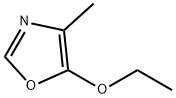
5417-35-6
7 suppliers
inquiry
![6,9-Epoxy[1,3]dioxepino[5,6-c]pyridine, 9-ethoxy-1,5,5a,6,9,9a-hexahydro-8-methyl-3-(1-methylethyl)-](/CAS/20210305/GIF/5205-63-0.gif)
5205-63-0
0 suppliers
inquiry

33140-27-1
18 suppliers
$60.00/25mg
![1,5-dihydro-3-isopropyl-8-methyl-[1,3]dioxepino[5,6-c]pyridin-9-ol](/CAS/GIF/1622-67-9.gif)
1622-67-9
7 suppliers
inquiry