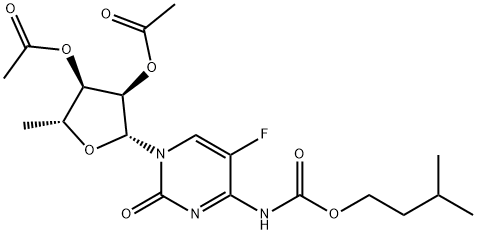
2’,3’-Di-O-acetyl-5'-deoxy-5-fluoro-N-[(3-methylbutoxy)carbonyl]cytidine synthesis
- Product Name:2’,3’-Di-O-acetyl-5'-deoxy-5-fluoro-N-[(3-methylbutoxy)carbonyl]cytidine
- CAS Number:162204-22-0
- Molecular formula:C19H26FN3O8
- Molecular Weight:443.42
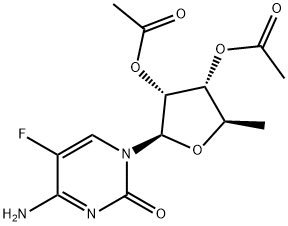
161599-46-8
448 suppliers
$6.00/250mg
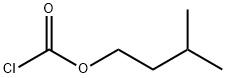
628-50-2
34 suppliers
inquiry
![2’,3’-Di-O-acetyl-5'-deoxy-5-fluoro-N-[(3-methylbutoxy)carbonyl]cytidine](/CAS/20180808/GIF/162204-22-0.gif)
162204-22-0
15 suppliers
$120.00/2mg
Yield:-
Reaction Conditions:
with potassium carbonate in acetone at 20 - 30;Inert atmosphere;
Steps:
6.1.2. Preparation of 5'-deoxy-5-fluoro-N4-(2-methyl-1-butyloxycarbonyl)cytidine
General procedure: To a solution of K2CO3 (0.626 g, 0.0453 mol) in acetone (80 mL) was added 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (14 g, 0.0425 mol) in a 500 mL 4-necked RB flask fitted with magnetic bar, thermo pocket, guard tube and nitrogen inlet and stirred for 10-15 min 2-Methyl-1-butyl chloroformate first half (2.279 g, 0.0151 mol) was added through additional funnel over a period of 15-20 min at room temperature and continued to stirred for 3 h. After completion of 3 h, second half of 3-methyl-1-butyl chloroformate (1.129 g, 0.0075 mol) added at 25-30 °C, progress of the reaction was monitored by TLC (ethylacetate: hexane 1:1). After completion of the reaction, the mixture was filtered and washed with 2 volumes (50 mL) of acetone. To the acetone layer 4 volumes of 10% NaOH solution added at -10 to -15 °C over 2 h. After completion of the reaction indicated by TLC, the pH of the reaction mixture adjusted to 6.0 by using concentrated HCl. The acetone was distilled off completely by using rota evaporator. Thus obtained aqueous layer extracted with ethyl acetate 5 volumes (50 mL) and ethyl acetate layer reduced to 1 volume stirred for 30 min then 5 volumes of tertiary methyl butyl ether (TBME) (50 mL) was added, the obtained precipitate was stirred for 1 h at 20-25 °C and 2 h at 0-5 °C filtered off washed with 9:1 volumes of TBME and ethyl acetate and suck dried to obtained the crude product and it was further purified by column chromatography (1:1 hexane/EtOAc) to get the title compound.
References:
Jhansi Rani;Raghavendra;Kishore;Nanda Kumar;Hema Kumar;Jagadeeswarareddy [European Journal of Medicinal Chemistry,2012,vol. 54,p. 690 - 696] Location in patent:experimental part