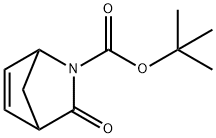
2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, 3-oxo-, 1,1-diMethylethyl ester synthesis
- Product Name:2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, 3-oxo-, 1,1-diMethylethyl ester
- CAS Number:162427-15-8
- Molecular formula:C11H15NO3
- Molecular Weight:209.24

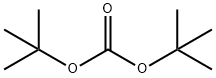
24424-99-5
824 suppliers
$13.50/25G
![(1S,4R)-2-Aza-bicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/130931-83-8.gif)
130931-83-8
167 suppliers
$8.00/100mg
![2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, 3-oxo-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/162427-15-8.gif)
162427-15-8
36 suppliers
inquiry
Yield: 80%
Reaction Conditions:
with dmap in tetrahydrofuran at 20;
Steps:
a [(±)-tert-butyl 3-oxo-2-azabicyclo(2.2.1)hept-5-ene-2-carboxylate] (2)
[(±)-tert-butyl 3-oxo-2-azabicyclo(2.2.1)hept-5-ene-2-carboxylate] (2). A solution of di-tert-butyl dicarbonate (110.0 g, 504.5 mmol) in tetrahydrofuran (50 ml) was added slowly to a suspension of racemic 1 (50.0 g, 403.2 mmol), and 4-dimethylaminopyridine (0.5 g, 4.0 mmol) in tetrahydrofuran (150 ml). The brown, hazy solution was stirred at 20°C until reaction was complete. The solution was concentrated in vacuo to give brown foam. Recrystalhsation twice from cyclohexane afforded the product 2 (racemic) as pale pink crystals (80.8 g, 80%); mp 70.5-71.5°C; *H NMR (CDC13): δ 1.5 (s, 5H), 2.15 (d, J = 8.5 Hz, 1H), 2.35 (d, J= 8.5 Hz, 1H), 3.39 (s, 1H), 4.96 (s, 1H), 6.66 (m, 1H), 6.89 (dd, J = 5.6 & 2.1 Hz, 1H); MS: (M+H)+ 210.
References:
UNIVERSITY OF GEORGIA RESEARCH FOUNDATION, INC.;CHU, Chung, K. WO2013/123138, 2013, A2 Location in patent:Page/Page column 32; sheet 2

24424-99-5
824 suppliers
$13.50/25G
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)
49805-30-3
363 suppliers
$5.00/5g
![2-Azabicyclo[2.2.1]hept-5-ene-2-carboxylic acid, 3-oxo-, 1,1-diMethylethyl ester](/CAS/20150408/GIF/162427-15-8.gif)
162427-15-8
36 suppliers
inquiry