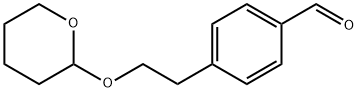
4 - (2 - ((Tetrahydro - 2H - pyran - 2 - yl)oxy)ethyl)benzaldehyde synthesis
- Product Name:4 - (2 - ((Tetrahydro - 2H - pyran - 2 - yl)oxy)ethyl)benzaldehyde
- CAS Number:163164-46-3
- Molecular formula:C14H18O3
- Molecular Weight:234.29
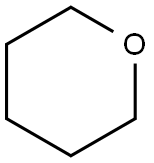
142-68-7
182 suppliers
$14.00/25mL
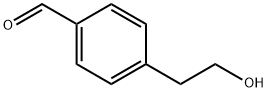
163164-47-4
51 suppliers
$156.56/250mg

163164-46-3
7 suppliers
inquiry
Yield:163164-46-3 60%
Reaction Conditions:
with tert.-butylhydroperoxide;ferrocene;phenylsilane at 20 - 80; for 12 h;Inert atmosphere;
Steps:
23 Example 23
Compound 23: Ferrocene (5.5 mg, 0.025 mmol) was added sequentially to a 25 mL two-necked flask,4-formyl alcohol (44.1 mg, 0.25 mmol),Phenyl silane (109 [mu] L, 0.75 mmol)Tert-butyl hydroperoxide (88 [mu] L, 0.75 mmol)The gas was replaced with dry N2 for 3 times,Finally, dry tetrahydropyran (2.0 mL) was added under N2.The mixture was stirred at room temperature and heated to 80 ° C to carry out the reaction,Until the thin layer chromatography monitoring of raw materials is completed.At the end of the reaction, 15.0 mL of NaCl solution was added at room temperature,Extracted with ether 15.0mL three times, combined with organic phase decompression steaming,The product was purified by column chromatography in 60% yield.
References:
CN107056732,2017,A Location in patent:Paragraph 0075; 0076; 0127; 0129
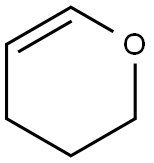
110-87-2
519 suppliers
$10.00/1g

163164-46-3
7 suppliers
inquiry
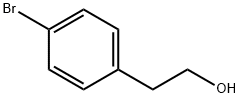
4654-39-1
273 suppliers
$5.00/1g

163164-46-3
7 suppliers
inquiry