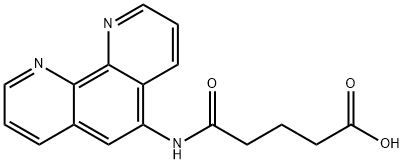
5-(1,10-Phenanthroline-5-ylaMino)-5-oxopentanoic acid synthesis
- Product Name:5-(1,10-Phenanthroline-5-ylaMino)-5-oxopentanoic acid
- CAS Number:163628-20-4
- Molecular formula:C17H15N3O3
- Molecular Weight:309.32

108-55-4
432 suppliers
$6.00/25g

54258-41-2
160 suppliers
$10.00/250mg

163628-20-4
8 suppliers
inquiry
Yield:163628-20-4 70.1%
Reaction Conditions:
Stage #1: 5-Amino-1,10-phenanthrolinewith methylene chloride;toluene-4-sulfonamide at 20; for 0.05 h;
Stage #2: dihydro-2H-pyran-2,6(3H)-dione at 60; for 72 h;Inert atmosphere;
Steps:
1 Example 1: Synthesis of Ligand L1 (C17H15N3O3)
Weigh 1,10-phenanthroline-5-amino (390 mg, 2 mmol),Add 60 mL of CH3Cl, stir at room temperature to fully dissolve,Add p-toluenesulfonamide (PTSA, 76 mg, 0.4 mmol),Stirred for 3 min at room temperature, when the solution changed from pale yellow to orange, glutaric anhydride (1.37 g, 12 mmol) was added, and under argon protection,Placed in a 60°C oil bath for reflux reaction for 72h,A white viscous solid was precipitated in the reaction solution. After the reaction was completed, it was cooled to room temperature and filtered to obtain a filter residue. After the filter residue was washed alternately with CH3Cl and ultrapure water for 3 times, it was filtered through a Buchner funnel.The obtained solid was dried to obtain a white solid product, yield: 70.1%; the 1H NMR spectrum of ligand L1 is shown in Figure 1, and the high-resolution mass spectrum is shown in Figure 4;
References:
CN114835706,2022,A Location in patent:Paragraph 0011