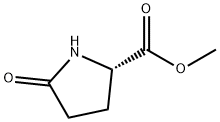
2-Pyrrolidinone,5-(3-methyl-5-isoxazolyl)-,(S)-(9CI) synthesis
- Product Name:2-Pyrrolidinone,5-(3-methyl-5-isoxazolyl)-,(S)-(9CI)
- CAS Number:163849-08-9
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18
Yield:163849-08-9 46%
Reaction Conditions:
with n-butyllithium;sulfuric acid;sodium carbonate in tetrahydrofuran;water;ethyl acetate;
Steps:
13.b 13b.
13b. 5(S)-(3-methyl-5-isoxazolyl)-2-pyrrolidinone To a cooled (0°-5° C.) solution of acetone oxime (11.74 g, 160.8 mmol) in THF (200 mL) was slowly added n-butyl lithium (128.6 mL, 2.5M, 321.6 mmol) in hexanes. After being stirred at 0°-5° C. for one hour, a solution of pyroglutamic methyl ester (10.0 g, ~69.9 mmol, the product of step 13a)in THF (50 mL) was added. After stirring for 4 hr, the solution was allowed to slowly warm up to room temperature, and the stirring was continued for 16 hr. Sulfuric acid (35 g, 98%) was slowly added with cooling, followed by the addition of water (35 mL). The resulting mixture was refluxed for one hour. The organic layer was decanted and the slurry was washed with ethyl acetate (5*50 mL). To the mixture was added ethyl acetate (400 mL) and sodium carbonate until basic. Again, the organic layer was decanted and the slurry was washed with ethyl acetate (4*20 mL). The combined organics were then dried over magnesium sulfate. Evaporation of the solvents gave the title product (5.33 g, 46%), which was taken directly to the next step. HPLC (analytical Chiralark AD column) analysis indicated >99.6% ee.
References:
US5424444,1995,A
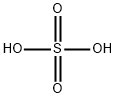
7664-93-9
6 suppliers
$23.30/1090731000

127-06-0
406 suppliers
$5.67/25g

163849-08-9
2 suppliers
inquiry

7664-93-9
6 suppliers
$23.30/1090731000
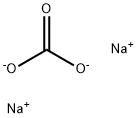
497-19-8
1383 suppliers
$14.00/1KG

127-06-0
406 suppliers
$5.67/25g

163849-08-9
2 suppliers
inquiry