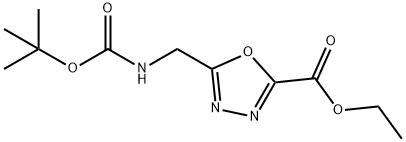
ethyl 5-({[(tert-butoxy)carbonyl]aMino}Methyl)- 1,3,4-oxadiazole-2-carboxylate synthesis
- Product Name:ethyl 5-({[(tert-butoxy)carbonyl]aMino}Methyl)- 1,3,4-oxadiazole-2-carboxylate
- CAS Number:164029-51-0
- Molecular formula:C11H17N3O5
- Molecular Weight:271.2698
164029-45-2
0 suppliers
inquiry
![ethyl 5-({[(tert-butoxy)carbonyl]aMino}Methyl)-
1,3,4-oxadiazole-2-carboxylate](/CAS/GIF/164029-51-0.gif)
164029-51-0
16 suppliers
$137.75/1g
Yield:164029-51-0 44%
Reaction Conditions:
with triethylamine;triphenylphosphine in tetrachloromethane;dichloromethane at 20; for 12.5 h;Heating / reflux;
Steps:
7.1
1.Ethyl 5-{[(tert-butoxy)carbonylamino]methyl}-1,3,4-oxadiazole-2-carboxylate (8) A solution of compound 7 (59 g, 200 mmol) and triethylamine (42 ml, 0.57 mol) in carbon tetrachloride (60 ml) is added to a stirred, room temperature of triphenylphosphine (63 g, 0.24 mol) in methylene chloride (900 ml).The reaction mixture is stirred for 30 minutes and then refluxed for 12 hours.After cooling to room temperature, the volatiles are removed in vacuo and the resulting residue is filtered through silica using methylene chloride:ethyl acetate (9:1) as the eluent.After removing the volatiles in vacuo, the crude product is purified by flash column chromatography over silica gel using methylene chloride:ethyl acetate (19:1) as the eluent. 24 g (44%) Of compound 8 is obtained as orange oil, which solidifies to a yellow solid on standing.
References:
US2004/19215,2004,A1 Location in patent:Page 6-7