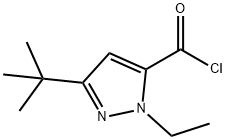
1H-Pyrazole-5-carbonyl chloride, 3-(1,1-dimethylethyl)-1-ethyl- (9CI) synthesis
- Product Name:1H-Pyrazole-5-carbonyl chloride, 3-(1,1-dimethylethyl)-1-ethyl- (9CI)
- CAS Number:167889-79-4
- Molecular formula:C10H15ClN2O
- Molecular Weight:214.69
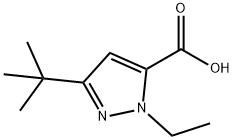
195447-83-7
13 suppliers
inquiry

167889-79-4
1 suppliers
inquiry
Yield:167889-79-4 84%
Reaction Conditions:
with thionyl chloride for 5 - 6 h;Product distribution / selectivity;Heating / reflux;
Steps:
5.A; 6.A
EXAMPLE 5; Preparation of 3- (1, 1-dimethylethyl)-l-ethyl-N- [3- (5-methyl-1, 2, 4-oxadiazol-3-yl) phenyl] - lH-pyrazole-5-carboxamide (Compound7); Step A: Preparation of 3-(1,1-dimethylethyl)-1-ethyl-1H-pyrazole-5-carbonyl chloride; A solution of 3- (l, l-dimethylethyl)-1-ethyl-lH-pyrazole-5-carboxylic acid (i. e., product of Example 1, Step D) (1.96 g, 10. 0 mmol) in thionyl chloride (99%,-5mL) was heated at reflux for about 6 h, and then allowed to cool to room temperature while being stirred overnight. The reaction mixture was then concentrated in vacuo Dichloromethane (No.10mL) was added to the residue, and the mixture was concentrated again; this was repeated twice to provide the title compound as a yellow liquid (1.80 g, 84% yield). 1H NMR (CDCl3) No. 6.935 (s, 1H), 4.40-4. 47 (m, 2H), 1.36-1. 57 (m, 3H), 1.31-1. 32 (s, 9H).; EXAMPLE 6; Preparation of 3- (1, 1-dimethylethyl)-1-ethyl-N- [3- (5-isoxazolyl) phenyl]-lH-pyrazole- 5-carboxamide (Compound 46); Step A: Preparation of N (3-acetylphenyl)-3- (1, 1-dimethylethyl)-1-ethyl- 1H-pyrazole-5-carboxamide; A solution of 3- (l, l-dimethylethyl)-1-ethyl-1H-pyrazole-5-carboxylic acid (i. e. the product of Example 1, Step D) (0.5 g, 2.54 mmol) in thionyl chloride (6 mL) was heated at reflux under N2 atmosphere for 5 h. The reaction mixture was concentrated under vacuum and then taken up in dichloromethane and concentrated again to provide the corresponding acid chloride as an oil. Triethylamine (533 pL, 3.82 mmol) was added to a solution of 1- (3-aminophenyl) ethanone (alternatively named 3'-aminoacetophenone, 361 mg, 2.67 mmol) in dichloromethane (6 mL) cooled to 0 °C under a N2 atmosphere. To this mixture a solution of the acid chloride in dichloromethane (2 mL) was added dropwise using an addition funnel. The reaction mixture was then allowed to stir at room temperature overnight, after which time it was diluted with dichloromethane and water. The layers were separated, and the organic layer was washed with water (lx), dried using an ExtubeTM (tube containing diatomaceous earth marketed by Varian, Inc. , 24201 Frampton Avenue, Harbor City, CA 90710 USA) and then concentrated to leave the title compound as an oil (0.84 g). 1H NMR (CDC13) 8 8.06 (s, 1H), 8.0 (d, 1H), 7.9 (br s, 1H, NH), 7.7 (d, 1H), 7.4 (t, 1H), 6.5 (s, 1H), 4.5 (q, 2H), 2.6 (s, 3H), 1.45 (t, 3H), 1.34 (s, 9H).
References:
WO2005/40152,2005,A1 Location in patent:Page/Page column 60; 62