N-BOC-4-(2-ETHOXY-2-OXOETHYL)-6H-THIENO[2,3-B]PYRROLE synthesis
- Product Name:N-BOC-4-(2-ETHOXY-2-OXOETHYL)-6H-THIENO[2,3-B]PYRROLE
- CAS Number:171513-17-0
- Molecular formula:C15H19NO4S
- Molecular Weight:309.38
Yield:171513-17-0 90%
Reaction Conditions:
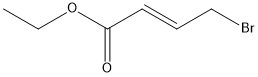
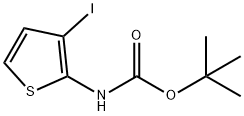
Stage #1: 4-bromocrotonic ethyl ester;N-(tert-butoxycarbonyl)-3-iodo-2-aminothiophenewith potassium carbonate in DMF (N,N-dimethyl-formamide) at 20; for 16 h;
Stage #2: with triphenylphosphine;palladium diacetate in DMF (N,N-dimethyl-formamide) at 70; for 8 h;
Steps:
3
EXAMPLE 3 Preparation of Ethyl 1-R6- (T-BUTOXVCARBONVL)-4-THIENOR2. 3-BLAVRROL-4-VLLACETATE A mixture of 3-IODO-2-[(N-T-BUTOXYCARBONYL) amino] thiophene (10 g, 32 mmol), K2CO3 (9 g, 2 equiv. ) and ethyl 4-bromocrotonate (9 g, 1.5 equiv. ) in DMF is stirred at room temperature for 16 h, treated with triphenylphosphine (838 mg, 0.1 equiv. ) and palladium acetate (358 mg, 0.05 equiv. ), heated at 70°C for 8 h, cooled to room temperature, diluted with water and extracted with EtOAc. The extracts are combined, dried over MGS04 and CONCENTRATED IN VACUO. The resultant residue is chromatographed (20% EtOAc in hexanes as eluent) to afford the title product as a clear oil, 8.6 g (90% yield), identified by HPLC and mass spectral analyses.
References:
WO2005/12311,2005,A1 Location in patent:Page/Page column 23
119485-56-2
25 suppliers
inquiry
![N-BOC-4-(2-ETHOXY-2-OXOETHYL)-6H-THIENO[2,3-B]PYRROLE](/CAS/20180527/GIF/171513-17-0.gif)
171513-17-0
7 suppliers
inquiry