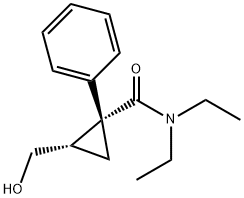
rac N,N-diethyl-2-(hydroxyMethyl)-1-phenyl-cyclopropanecarboxaMide synthesis
- Product Name:rac N,N-diethyl-2-(hydroxyMethyl)-1-phenyl-cyclopropanecarboxaMide
- CAS Number:172016-06-7
- Molecular formula:C15H21NO2
- Molecular Weight:247.33
![1-Phenyl-3-oxabicyclo[3.1.0]hexan-2-one](/CAS/GIF/63106-93-4.gif)
63106-93-4
137 suppliers
$7.00/1g

172016-06-7
8 suppliers
$95.00/5mg
Yield:172016-06-7 66.8%
Reaction Conditions:
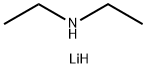
Stage #1: diethylaminewith n-butyllithium in tetrahydrofuran;hexane at -75; for 1 h;
Stage #2: 1-phenyl-3-oxa-bicyclo[3.1.0]hexan-2-one in tetrahydrofuran;hexane at -78 - -60;
Stage #3: with water;ammonium chloride in tetrahydrofuran;hexane;
Steps:
2
Referential Example 2: Production of (Z)-1-phenyl-1-diethylaminocarbonyl-2-hydroxymethylcyclopropane After cooling a mixed solution of diethylamine (8.1 kg,111 mol) and tetrahydrofuran (33.2 kg) down to -75°C, 17% n-butyllithium-hexane solution (40.4 kg, 108 mol) was added dropwise into the above mixture and then stirred for 1 hour. Thereafter, a solution of 2-oxo-1-phenyl-3-oxabicyclo[3.1.0]hexane(12.4 kg, 71.2 mol) dissolved with tetrahydrofuran (10.4 kg) added dropwise into the mixture and then the resulting mixture was stirred at -60 to -78°C. After completion of the reaction, the reacted liquid was added dropwise into 20% aqueous solution of ammonium chloride (43.2 kg). After subjecting to phase separation, the organic layer was concentrated by about 40% by weight under a reduced pressure. Toluene (43.1 kg) was added to the concentrated residue, and then the added mixture was washed twice with water. The organic layer obtained was concentrated under a reduced pressure to obtain 11.5 kg of light-yellow oily substance as the titled compound. Yield is 66.8%. The obtained oily substance of (Z)-1-phenyl-1-diethylaminocarbonyl-2-hydroxymethylcyclopropane was crystallized with a mixed solvent of ethyl acetate and n-heptane to obtain light yellow crystals.
References:
EP1770084,2007,A1 Location in patent:Page/Page column 4
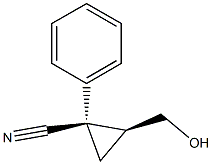
910028-42-1
2 suppliers
inquiry

172016-06-7
8 suppliers
$95.00/5mg
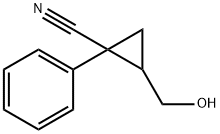
60788-55-8
3 suppliers
inquiry

172016-06-7
8 suppliers
$95.00/5mg

![(1R,5S)-1-phenyl-3-oxabicyclo[3.1.0]hexan-2-one](/CAS/20180601/GIF/96847-52-8.gif)