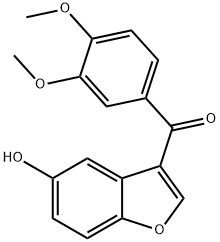
(3,4-Dimethoxy-phenyl)-(5-hydroxy-benzofuran-3-yl)-methanone synthesis
- Product Name:(3,4-Dimethoxy-phenyl)-(5-hydroxy-benzofuran-3-yl)-methanone
- CAS Number:17249-66-0
- Molecular formula:C17H14O5
- Molecular Weight:298.29
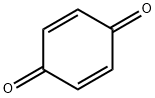
106-51-4
476 suppliers
$10.00/5g

127172-22-9
21 suppliers
$85.00/250mg

17249-66-0
4 suppliers
inquiry
Yield:17249-66-0 92%
Reaction Conditions:
with Amberlyst-15in PEG 400 at 20; for 0.166667 h;Green chemistry;
Steps:
3.1. General Procedure for the Synthesis of 3-benzoyl-5-hydroxybenzofuran and Naphtho[1,2-b]furan Derivatives (3a-r)
General procedure: To a mixture of 2a (1 mmol), benzoquinone (BQ, 1 mmol) was added Amberlyst-15 (10 mol%) followed by PEG-400 (2mL) as a solvent and kept under stirring at room temperature for 10-15min. The progress of the reaction was monitored by using TLC. Upon completion of the reaction, the reaction mixture was cooled in a dry ice-acetone bath to precipate the PEG-400 and was extracted with ether (3x5 mL). PEG-400 being insoluble in ether, the ether layer was separated, dried over anhydrous MgSO4 and concentrated under reduced pressure to produce the crude product. The recovered PEG-400 phase along with the catalyst was reused for consecutive runs. The crude product thus obtained was subjectedto purification by column chromatography on silica gel (100-200 mesh size) using 10% ether acetate-petroleum ether aseluent to afford the desired product 3a.
References:
Bathula, Surendra Bose;Khagga, Mukkanti;Venkatasubramanian, Hariharakrishnan [Letters in Organic Chemistry,2017,vol. 14,# 5,p. 353 - 360]
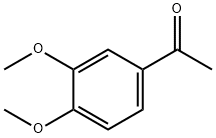
1131-62-0
316 suppliers
$14.00/5g

17249-66-0
4 suppliers
inquiry