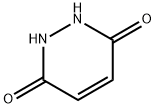
3-(3-Hydroxy-6-oxo-6H-pyridazin-1-yl)-propionitrile synthesis
- Product Name:3-(3-Hydroxy-6-oxo-6H-pyridazin-1-yl)-propionitrile
- CAS Number:17285-16-4
- Molecular formula:C7H7N3O2
- Molecular Weight:165.15
Yield:17285-16-4 7.06 g
Reaction Conditions:
with sodium hydroxide in ethanol at 100; for 14 h;Michael Addition;
Steps:
23 Example 23: Synthesis of dicamba ester 34a via Michael addition of acrylonitrile to maleic hydrazide
[ 0220 ] 44.8 g of maleic hydrazide (0.40 mol, Aldrich), 23.3 g of acrylonitrile (0.44 mol, Aldrich) and 300 ml of absolute ethanol were combined in a 500 ml flask equipped with a stirbar. 8 drops of 2.5N NaOH were added. The flask was immersed in a 100°C oil bath and a reflux condenser attached. After 14 hours of reaction, the mixture was filtered hot to remove some suspended white solid. Precipitation of a white solid from the filtrate began immediately thereafter. The flask was allowed to stand in a cold room to complete precipitation. [ 0221 ] The solid was recovered by filtration and dried at 80°C under 24" Hg (81.3 kPa) vacuum with nitrogen purge. In order to clean up residual water and eliminate the sodium salts, the product was suspended in a mixture of 10 g of acetic acid and 75 ml of water and stirred briefly. The solid was recovered by filtration, rinsed with methyl-i-butyl ether and acetone, and dried at 80°C under 24" Hg (81.3 kPa) vacuum with nitrogen purge. All of the dry product (7.06 g, 43 mmol) was combined with 4.3 g of triethylamine (1.0 equiv), 0.16 g of DMAP (3 mol %), 100 ml of CH2C12, and 11.3 g of dicamba acid chloride (1.1 equiv.) in a roundbottom flask equipped with a stirbar.
References:
WO2013/55944,2013,A2 Location in patent:Paragraph 0220; 0221