
Benzenepropanoic acid, 4-methoxy-3-(3-methoxypropoxy)-a-(1-methylethyl)-, (aR)- synthesis
- Product Name:Benzenepropanoic acid, 4-methoxy-3-(3-methoxypropoxy)-a-(1-methylethyl)-, (aR)-
- CAS Number:172900-71-9
- Molecular formula:C17H26O5
- Molecular Weight:310.39

1351987-18-2
0 suppliers
inquiry

172900-71-9
25 suppliers
$503.80/5MG
Yield:96.2 % ee
Reaction Conditions:
Stage #1: butyl-2-(4-methoxy-3-(3-methoxypropoxy)benzyl)-3-methylbutanoatewith hydrogenchloride;HLAP;water;2-amino-2-hydroxymethyl-1,3-propanediol at 40; for 48 h;Enzymatic reaction;
Stage #2: with citric acid in water at 20; pH=3.5;
Steps:
2
Enzymatic hydrolysis of compound (XI I) to supply the compound of formula (XI I I), (f?)-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-3-methylbutanoic acid.A 1 00-ml flask equipped with an efficient magnetic stirrer is charged with 1 .1 34 g of methyl-2-(4-methoxy-3-(3-methoxypropoxy)benzyl)-3-methylbutanoate (3.5 mmol) obtained by saponification and esterification with methanol of the butyl ester obtained in the preceding example; then 85 ml of a 0.3 M buffer solution of tris(hydroxymethyl)aminomethane/HCI with pH 7.5 are added. The mixture is stirred at high speed producing a finely dispersed suspension. 1 .750 g of HLAP (Aldrich) are added; the flask is stoppered and immersed in a thermostatic bath at 40 °C, and the suspension is stirred rapidly for 48 hours, monitoring the progress of the reaction by TLC (eluent hexane:ethyl acetate 8:2 mixture containing 3% of acetic acid; Rf ester = 0.34, Rf acid = 0.2; visualization after development with cerium ammonium molybdate). The mixture is cooled to room temperature and the pH is adjusted to 3.5 by adding 0.5 M solution of citric acid. After adding 1 0 ml of ethyl ether, the aqueous phase is saturated with NaCI and the mixture is stirred vigorously for 1 0 min, then filtered on Celite and washed with an ethyl ethenmethanol 9:1 mixture.The filtrate is transferred to a separatory funnel, the phases are separated and the aqueous phase is re-extracted with ethyl ether.The organic phases are combined and dried over sodium sulphate, then concentrated until a residue is obtained, which is purified by flash chromatography with a hexane:ethyl acetate elution gradient from 7:3 to 0:1 0.521 mg of acid are obtained, which is esterified with diazomethane and analysed by HPLC, showing an enantiomeric excess (e.e.) of 83.3%. The ester (475 mg) is analysed in its turn by HPLC, showing e.e. of 95.0%. The HPLC analyses are carried out in the following conditions:- column : Chiralpak AD 250 x 4.6 mm ;- flow rate: 1 ml/min ; - injected volume: 10 μΙ;- detector wavelength: 210 nm;- column temperature: RT;- isocratic mobile phase, 95% hexane, 5% ethanol, 0.1 % trifluoroacetic acid;- retention times: (S)-(-)-acid 1 1 .17 min, (f?)-(+)-acid 13.03 min.The degree of conversion (evaluated with 1 H NMR) is equal to 50.6%. The enantioselectivity, E, of the mixture is also measured, and is found to be 40.3. The enantioselectivity is defined as:E = In {(1 -ees)(eep)/ees+eep} / In {(1 +ees)(eep)/ees+eep}The procedure of this example is repeated on ethyl-2-(4-methoxy-3-(3- methoxypropoxy)benzyl)-3-methylbutanoate and / butyl-2-(4-methoxy-3-(3- methoxypropoxy)benzyl)-3-methylbutanoate, giving the following results:The enantiomeric excess increased to values above 99.5% by formation of the ammonium salt in isopropanol, filtration, hydrolysis with dilute HCI and extraction with toluene.
References:
WO2011/151442,2011,A2 Location in patent:Page/Page column 20-21
![2-Isopropyl-3-[4-methoxy-3-(3-methoxypropoxy)phenyl]acrylic acid](/CAS/20180808/GIF/387868-07-7.gif)
387868-07-7
31 suppliers
$380.00/500mg

172900-71-9
25 suppliers
$503.80/5MG
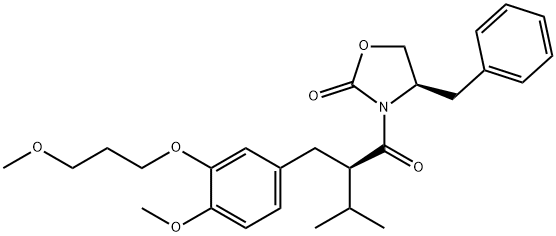
172900-72-0
4 suppliers
inquiry

172900-71-9
25 suppliers
$503.80/5MG
![2-Isopropyl-3-[4-methoxy-3-(3-methoxypropoxy)phenyl]acrylic acid](/CAS/20180808/GIF/387868-07-7.gif)
387868-07-7
31 suppliers
$380.00/500mg

172900-71-9
25 suppliers
$503.80/5MG
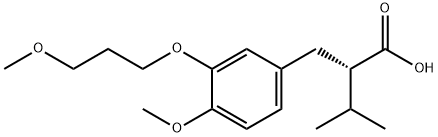
173433-57-3
3 suppliers
inquiry

173400-44-7
2 suppliers
inquiry

172900-71-9
25 suppliers
$503.80/5MG