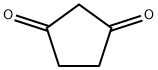
5H-Cyclopenta[b]pyridin-5-one,6,7-dihydro-2-methyl-(9CI) synthesis
- Product Name:5H-Cyclopenta[b]pyridin-5-one,6,7-dihydro-2-methyl-(9CI)
- CAS Number:173064-91-0
- Molecular formula:C9H9NO
- Molecular Weight:147.1739
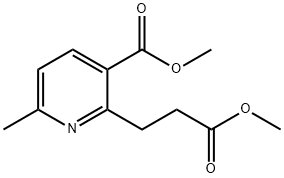
853179-88-1
0 suppliers
inquiry
![5H-Cyclopenta[b]pyridin-5-one,6,7-dihydro-2-methyl-(9CI)](/CAS/20180808/GIF/173064-91-0.gif)
173064-91-0
2 suppliers
inquiry
Yield:173064-91-0 66.1%
Reaction Conditions:
Stage #1: methyl 2-(2-(methoxycarbonyl)ethyl)-6-methylpyridine-3-carboxylatewith sodium methylate in tetrahydrofuran; for 2 h;Inert atmosphere;Reflux;
Stage #2: with hydrogenchloride in water; for 2 h;Reflux;
Steps:
d d) 2-Methyl-6,7-dihydro-5H-cyclopenta[b]pyridin-5-one:
Sodium methoxide (0.956 g, 17.70 mmol) was added to a solution of methyl 2-(3-methoxy-3- oxopropyl)-6-methylnicotinate (2.8 g, 11.80 mmol) in THF (30 mL) under nitrogen atmosphere. The reaction mixture was warmed to reflux during 2 h. The solvent was removed under vacuo and HC1 (20 ml, 90 mmol) 4.5 M was added, the mixture was stirred 2 h at reflux. The reaction mixture was dissolved in water and pH was adjusted to ~8.0 by adding K2CO3 before extraction of the product with CH2CI2. The organic layer was washed with water and brine solution, dried over anhydrous Na2S04. Filtrate was evaporated completely under reduced pressure to give crude residue. The crude residue was purified by silica gel column chromatography by using EtOAc in hexane as eluent, the product was eluted at 40% EtO Ac/Pet-ether to get 2-methyl-6,7- dihydro-5H-cyclopenta[b]pyridin-5-one (1.3 g, 66.1% yield) as a colorless oil. 1H NMR (400 MHz, CDCI3): d 7.91 (d, / = 8.4 Hz, 1H), 7.19 (d, /= 7.6 Hz, 1H), 3.24 (t, J= 6.0 Hz, 2H), 2.78 (t, J= 8.0 Hz, 2H), 2.67 (s, 3H). LCMS (ES) m/z 147.98 (M+H)+
References:
WO2019/149959,2019,A1 Location in patent:Page/Page column 60; 62
![3-Pyridinecarboxylic acid, 2-[(1E)-3-methoxy-3-oxo-1-propen-1-yl]-6-methyl-, methyl ester](/CAS/20210305/GIF/853179-76-7.gif)
853179-76-7
0 suppliers
inquiry
![5H-Cyclopenta[b]pyridin-5-one,6,7-dihydro-2-methyl-(9CI)](/CAS/20180808/GIF/173064-91-0.gif)
173064-91-0
2 suppliers
inquiry
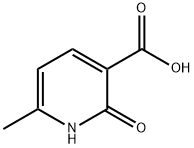
38116-61-9
184 suppliers
$10.00/1g
![5H-Cyclopenta[b]pyridin-5-one,6,7-dihydro-2-methyl-(9CI)](/CAS/20180808/GIF/173064-91-0.gif)
173064-91-0
2 suppliers
inquiry
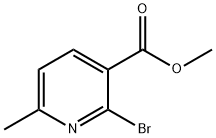
885277-48-5
54 suppliers
$33.00/100mg
![5H-Cyclopenta[b]pyridin-5-one,6,7-dihydro-2-methyl-(9CI)](/CAS/20180808/GIF/173064-91-0.gif)
173064-91-0
2 suppliers
inquiry