
(R)-benzyl 1-(4-(1H-pyrrolo[2,3-b]pyridin-4-ylcarbaMoyl)phenyl)ethylcarbaMate synthesis
- Product Name:(R)-benzyl 1-(4-(1H-pyrrolo[2,3-b]pyridin-4-ylcarbaMoyl)phenyl)ethylcarbaMate
- CAS Number:173897-93-3
- Molecular formula:C24H22N4O3
- Molecular Weight:414.46
![Carbamic acid, [1-?[4-?(chlorocarbonyl)?phenyl]?ethyl]?-?, phenylmethyl ester, (R)?- (9CI)](/CAS/20210111/GIF/173897-73-9.gif)
173897-73-9
2 suppliers
inquiry
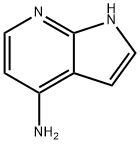
74420-00-1
143 suppliers
$46.00/100mg
![(R)-benzyl 1-(4-(1H-pyrrolo[2,3-b]pyridin-4-ylcarbaMoyl)phenyl)ethylcarbaMate](/CAS/GIF/173897-93-3.gif)
173897-93-3
17 suppliers
$498.88/5MG
Yield:173897-93-3 330 mg
Reaction Conditions:
Stage #1: (R)-4-(1-benzyloxycarbonylaminoethyl)benzoic acid chloride;4-amino-1H-pyrrolo<2,3-b>pyridinewith N-ethyl-N,N-diisopropylamine in acetonitrile at 20; for 8 h;
Stage #2: with sodium methylate in methanol at 20; for 0.5 h;
Steps:
1.a
Then, the crystals were dissolved in acetonitrile (10 mL) and added dropwise to a solution of 4-amino-1H-pyrrolo[2,3-b]pyridine (240 mg) and diisopropylethylamine (520 mg) in acetonitrile (10 mL), and the mixture was stirred at room temperature for 8 hrs.
The precipitated crystals were collected by filtration, dried and dissolved in methanol (7 mL).
Sodium methoxide (60 mg) was added and the mixture was stirred at room temperature for 30 min.
After the reaction, the mixture was concentrated under reduced pressure.
Water was added to the obtained residue and the mixture was extracted with ethyl acetate.
The extract was dried and the solvent was evaporated under reduced pressure.
The obtained crystals were washed with ethyl acetate to give (R)-N-(1H-pyrrolo[2,3-b]pyridin-4-yl)-4-(1-benzyloxycarbonylaminoethyl)benzamide (330 mg). PMR (DMSO-d6/TMS) δ: 1.33-1.40 (3H, m), 4.72-4.78 (1H, m), 4.98-5.04 (2H, m), 6.78-6.82 (1H, m), 7.32-8.16 (13H, m)
References:
EP1378247,2016,B1 Location in patent:Paragraph 0076